Question
The set-up below represents apparatus that may be used to separate a mixture of two miscible liquids "C" and "D" whose boiling points are 80°C and 100°C respectively.
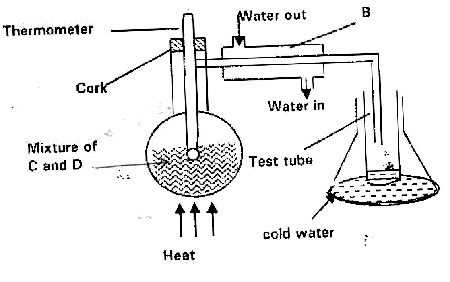
(a) Name B.
(b) What is the purpose of the thermometer?
(c) Which liquid is collected in the test tube?

(a) Name B.
(b) What is the purpose of the thermometer?
(c) Which liquid is collected in the test tube?
Answer
(a) Liebig's condenser
(b) Determine the point at which one of the liquids in a mixture has evaporated completely. Temperature tends to remain constant when one liquid in a mixture is evaporating.
(c) Liquid 'C' since it is more volatile.
(b) Determine the point at which one of the liquids in a mixture has evaporated completely. Temperature tends to remain constant when one liquid in a mixture is evaporating.
(c) Liquid 'C' since it is more volatile.

