| Name | Formula | Oxidation state of N | Ratio of N:O |
|---|---|---|---|
| Nitrogen Oxide or nitrous oxide |
NO | +2 | 1:1 |
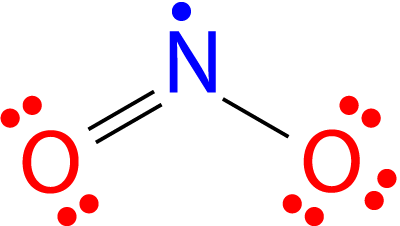
| Nitrogen dioxide | NO2 | +4 | 1:2 |
| Nitrous oxide (Laughing gas) |
N2O | +1 | 2:1 |
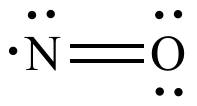
3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O

2NO → N2 + O2 (at high temperature)
From the equation above we can see that once the decomposition starts 50% O2 gets evolved and this O2 supports combustion thus making the reaction more violent.SO2 + 2NO + H2O → H2SO4 + N2O
2) NO acts as a reducing agent3I2 + 2NO + 4H2O → 2HNO3 + 6HI
3) With halogens NO can form addition compounds as:2NO + Cl2 → 2NOCl (NOCl is nitrosyl chloride)
It reacts in the same way with fluorine and bromine.
FeSO4 + 5H2O + NO → [Fe(H2O)5NO]SO4
penta aqua nitrosyl iron (II) sulphate
2Pb(NO3)2 → 2PbO + 4NO2 + O2
Please Note: Hydrated nitrate salts on heating react violently and explode.2NO2 + H2O → HNO2 + HNO3
2) With hot water the reaction is3NO2 + H2O → 2HNO3 + NO
3) Being acidic it reacts with bases as2NO2 + 2KOH → KNO3 + H2O + KNO2
4) It is also a strong oxidising agent.H2S + 3NO2 → 3NO + H20 + S02
5) With excess oxygen and water NO2 gives HNO3.4NO2 + O2 + 2H2O → 4HNO3
6) It reacts with concentrated H2SO4 to give nitrosyl hydrogen sulphate2NO2 + H2SO4 → SO2(OH)ONO + HNO3
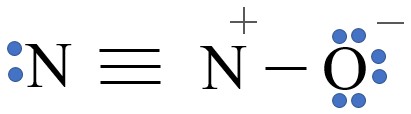
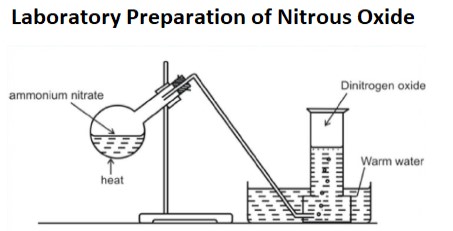
NH4NO3 → N2O + 2H2O
(endothermic reaction)
2N2O → 2N2 + O2
If a glowing piece of Mg, Cu, or P is introduced in such an environment, these pieces burn brightly due to the O2 produced from decomposition of N2O.
2N2O + 2Na → Na2O + 2N2
Na2O is sodium peroxide