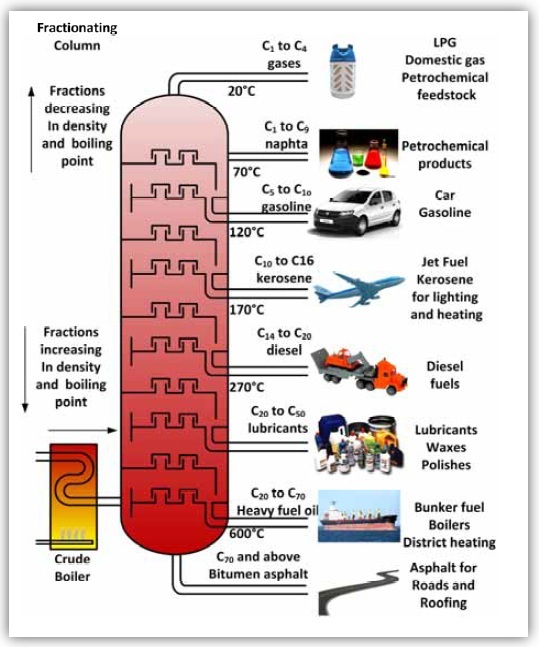
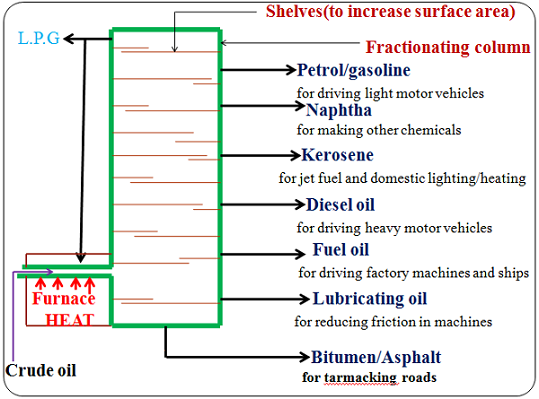
Crude oil is heated until it boils and then the hydrocarbon gases are entered into the bottom of the fractionating column.
As the gases go up the column the temperature decreases.
The hydrocarbon gases condense back into liquids and the fractions are removed from the sides of the column.
The smaller the hydrocarbon molecule, the further it rises up the column before condensing.
The fractionating column operates continuously.
The temperatures shown are approximate.
A sample of crude oil may be separated in the laboratory by fractional distillation.
The collection vessel is changed as the temperature rises to collect the different fractions.
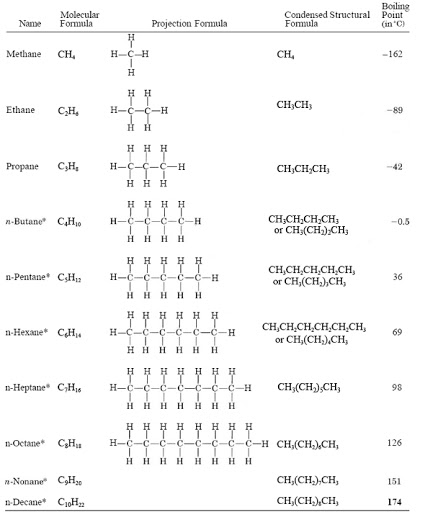
Isomers of Alkanes
Isomers are compounds with the same molecular general formula but different molecular structural formula.
Isomerism is the existence of a compounds having the same general/molecular formula but different structural formula.
The first three alkanes do not form isomers.
Isomers are named by using the
IUPAC(International Union of Pure and Applied Chemistry) system of nomenclature /naming.
The IUPAC system of nomenclature uses the following basic rules/guidelines;
- Identify the longest continuous carbon chain to get/determine the parent alkane.
- Number the longest chain form the end of the chain that is near the branches so as the branch get the lowest number possible.
- Determine the position, number and type of branches.
- Name them as methyl, ethyl, propyl e.tc. according to the number of carbon chains attached to the parent alkane.
- Name them fluoro-,chloro-,bromo-,iodo- if they are halogens.
- Use prefix di-,tri-,tetra-,penta-,hexa- to show the number of branches attached to the parent alkane.
Occurrence and Extraction of Alkanes
- Crude oil ,natural gas and biogas are the main sources of alkanes.
- Natural gas is found on top of crude oil deposits and consists mainly of methane.
- Biogas is formed from the decay of waste organic products like animal dung and cellulose.
- When the decay takes place in absence of oxygen , 60-75% by volume of the gaseous mixture of methane gas is produced.
- Crude oil is a mixture of many flammable hydrocarbons/substances.