Organic chemistry is a subdiscipline of chemistry that studies the structure, properties and reactions of organic compounds, which contain carbon in covalent bonding.
Read More On
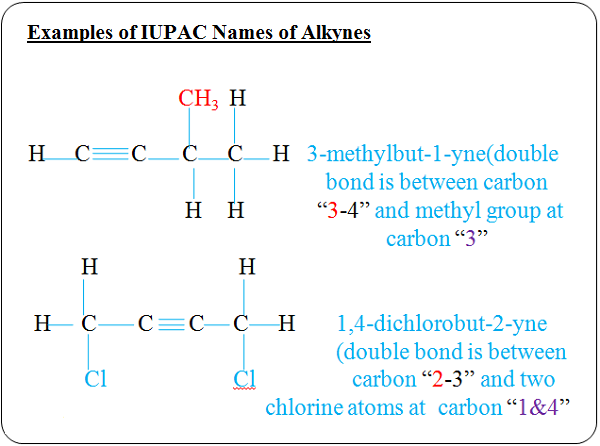
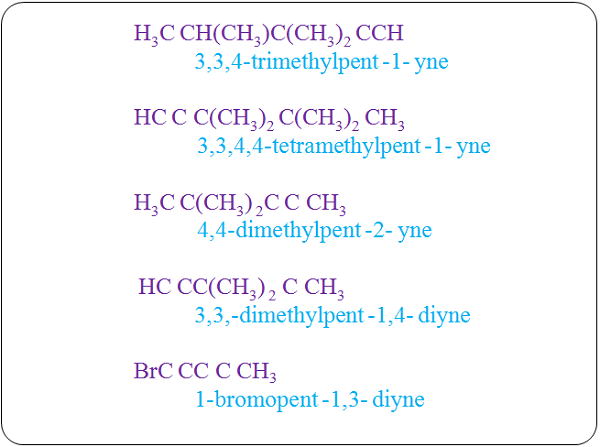
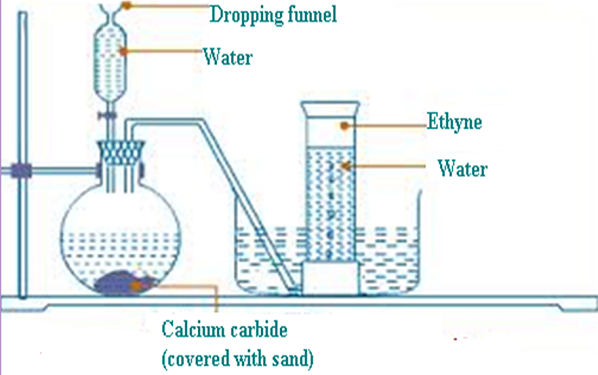
CaC2(s) + 2H2O(l) → Ca(OH)2 (aq) + C2H2 (g)
Alkyne + Air → Carbon (IV) Oxide + Water
2C2H2(g)+ 5O2(g) → 4CO2(g) + 2H2O(l/g)
Alkyne + Air → Carbon (II) Oxide/Carbon + Water
2C2H2(g)+ O2(g) → 2CO2(g) + C + 2H2O(l/g)