- An acid may be defined as a substance that turn litmus red.
- A base may be defined as a substance that turn litmus blue.
- Litmus is a lichen found mainly in West Africa.
- It changes its colour depending on whether the solution it is in, is basic/alkaline or acidic.
- It is thus able to identify/show whether.
1. An acid is a substance that dissolves in water to form H+/H3O+ as the only positive ion/cation.
- This is called the Arrhenius definition of an acid.- From this definition, an acid dissociate/ionize in water releasing H+ thus:
HCl(aq) → H+ (aq) + Cl- (aq)
HNO3(aq) → H+ (aq) + NO3-(aq)
CH3COOH(aq) → H+ (aq) + CH3COO-(aq)
H2SO4(aq) → 2H+ (aq) + SO42-(aq)
H2CO3(aq) → 2H+ (aq) + CO32-(aq)
H3PO4(aq) → 3H+ (aq) + PO43-(aq)
HNO3(aq) → H+ (aq) + NO3-(aq)
CH3COOH(aq) → H+ (aq) + CH3COO-(aq)
H2SO4(aq) → 2H+ (aq) + SO42-(aq)
H2CO3(aq) → 2H+ (aq) + CO32-(aq)
H3PO4(aq) → 3H+ (aq) + PO43-(aq)
2. A base is a substance which dissolves in water to form OH- as the only negatively charged ion/anion.
- This is called Arrhenius definition of a base.
- From this definition, a base dissociate/ionize in water releasing OH- thus:
KOH(aq) → K+(aq) + OH-(aq)
NaOH(aq) → Na+(aq) + OH-(aq)
NH4OH(aq) → NH4+(aq) + OH-(aq)
Ca(OH)2(aq) → Ca2+(aq) + 2OH-(aq)
Mg(OH)2(aq) → Mg2+(aq) + 2OH-(aq)
NaOH(aq) → Na+(aq) + OH-(aq)
NH4OH(aq) → NH4+(aq) + OH-(aq)
Ca(OH)2(aq) → Ca2+(aq) + 2OH-(aq)
Mg(OH)2(aq) → Mg2+(aq) + 2OH-(aq)
3. An acid is a proton donor.
- A base is a proton acceptor.
- This is called Bronsted-Lowry definition of acids and bases.
- From this definition, an acid donates H+.
- H+ has no electrons and neutrons .It contains only a proton.
Examples
I. From the equation:-
HCl(aq) + H2O(l) === H3O+(aq) + Cl- (aq)
(a)
(i) For the forward reaction from left to right, H2O gains a proton to form H3O+ and thus H2O is a proton acceptor. It is a Bronsted-Lowry base.
(ii) For the backward reaction from right to left, H3O+ donates a proton to form H2O and thus H3O+ is an ‘opposite’ proton donor. It is a Bronsted-Lowry conjugate acid.
(b)
(i) For the forward reaction from left to right, HCl donates a proton to form Cl- and thus HCl is a proton donor. It is a Bronsted-Lowry acid.
(ii) For the backward reaction from right to left, Cl- gains a proton to form HCl and thus Cl- is an ‘opposite’ proton acceptor.It is a Bronsted-Lowry conjugate base.
II. From the equation:
-
HCl(aq) + NH3(aq) === NH3+(aq) + Cl- (aq)
(a)
(i) For the forward reaction from left to right, NH3 gains a proton to form NH4+ and thus NH3 is a proton acceptor.
It is a Bronsted-Lowry base.
(ii) For the backward reaction from right to left, NH4+ donates a proton to form NH3 and thus NH4+ is an ‘opposite’ proton donor.
It is a Bronsted-Lowry conjugate acid.
(b)
(i) For the forward reaction from left to right, HCl donates a proton to form Cl- and thus HCl is a proton donor. It is a Bronsted-Lowry acid.
(ii) For the backward reaction from right to left, Cl- gains a proton to form HCl and thus Cl- is an ‘opposite’ proton acceptor. It is a Bronsted-Lowry conjugate base.
4. Acids and bases show acidic and alkaline properties/characteristics only in water but not in other solvents e.g.
(a)Hydrogen chloride gas dissolves in water to form hydrochloric acid Hydrochloric acid dissociates/ionizes in water to free H+(aq)/H3O+(aq) ions. The free H3O+(aq) / H+(aq) ions are responsible for:- Turning blue litmus paper/solution red.
- Show pH value 1/2/3/4/5/6.
- Are good electrolytes/conductors of electricity/undergo electrolysis.
- React with metals to produce / evolve hydrogen gas and a salt. i.e.
Ionically:
For a monovalent metal: 2M(s) + 2H+(aq) → 2M+(aq) + H2(g)For a divalent metal: M(s) + 2H+(aq) → M2+(aq) + H2(g)
For a trivalent metal: 2M(s) + 6H+(aq) → 2M3+(aq) + 3H2(g)
Examples:
For a monovalent metal: 2Na(s) + 2H+(aq) → 2Na+(aq) + H2(g)
For a divalent metal: Ca(s) + 2H+(aq) → Ca2+(aq) + H2(g)
For a trivalent metal: 2Al(s) + 6H+(aq) → 2Al3+(aq) + 3H2(g)
5. React with metal carbonates and hydrogen carbonates to produce /evolve carbon(IV)oxide gas ,water and a salt. i.e.
Ionically:
For a monovalent metal:M2CO3(s)+ 2H+(aq) → 2M+(aq) + H2O (l)+ CO2(g)
MHCO3(s)+ H+(aq) → M+(aq) + H2O (l)+ CO2(g)
For a divalent metal: MCO3(s)+ 2H+(aq) → M2+(aq) + H2O (l)+ CO2(g)
M(HCO3)2(aq)+2H+(aq) →M2+(aq)+2H2O(l)+2CO2(g)
Examples:
For a monovalent metal: K2CO3(s)+ 2H+(aq) → 2K+(aq) + H2O (l)+ CO2(g)
NH4HCO3(s)+ H+(aq) → NH4+(aq) + H2O (l)+ CO2(g)
For a divalent metal: ZnCO3(s)+ 2H+(aq) → Zn2+(aq) + H2O (l)+ CO2(g)
Mg(HCO3) 2(aq)+2H+(aq) →Mg2+(aq)+2H2O(l)+2CO2(g)
(vi)neutralize metal oxides/hydroxides to salt and water only. i.e.
Ionically:
For a monovalent metal: M2O(s) + 2H+(aq) → 2M+(aq) + H2O (l)MOH(aq) + H+(aq) → M+(aq) + H2O (l)
For a divalent metal: MO(s) + 2H+(aq) → M2+(aq) + H2O (l)
M(OH) 2(s) + 2H+(aq) → M2+(aq) + 2H2O(l)
For a trivalent metal: M2O3(s) + 6H+(aq) → 2M3+(aq) + 3H2O (l)
M(OH) 3(s) + 3H+(aq) → M3+(aq) + 3H2O(l)
Examples:
For a monovalent metal: K2O(s) + 2H+(aq) → 2K+(aq) + H2O (l)
NH4OH(aq) + H+(aq) → NH4+(aq) + H2O (l)
For a divalent metal: ZnO (s) + 2H+(aq) → Zn2+(aq) + H2O (l)
Pb(OH) 2(s) + 2H+(aq) → Pb2+(aq) + 2H2O(l)
(b) Hydrogen chloride gas dissolves in methylbenzene /benzene but does not dissociate /ionize into free ions.
It exists in molecular state showing none of the above properties.
(c) Ammonia gas dissolves in water to form aqueous ammonia which dissociate/ionize to free NH4+ (aq) and OH-(aq) ions.
This dissociation/ionization makes aqueous ammonia to:
-
(i) turn litmus paper/solution blue.
(ii) have pH 8/9/10/11.
(iii) be a good electrical conductor.
(iv) react with acids to form ammonium salt and water only.
NH4OH(aq) + HCl(aq) → NH4Cl(aq) + H2O(l)
6. Solvents are either polar or non-polar.
A polar solvent is one which dissolves ionic compounds and other polar solvents.Water is polar solvent that dissolves ionic and polar substance by surrounding the free ions as below:
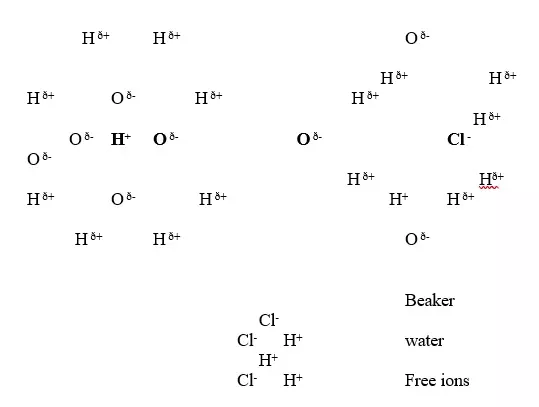
Oxygen atom is partially negative and two hydrogen atoms which are partially positive.
They surround the free H+ and Cl- ions.
A non polar solvent is one which dissolved non-polar substances and covalent compounds.
If a polar ionic compound is dissolved in non-polar solvent ,it does not ionize/dissociate into free ions as below:
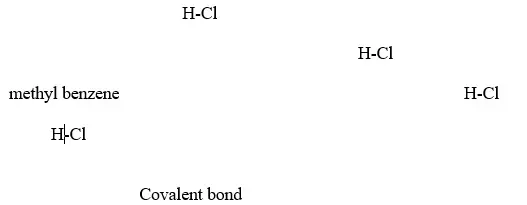
7. Some acids and bases are strong while others are weak.
(a) A strong acid/base is one which is fully/wholly/completely dissociated / ionized into many free H+ /OH- ions i.e.
I. Strong acids exists more as free H+ ions than molecules. e.g.
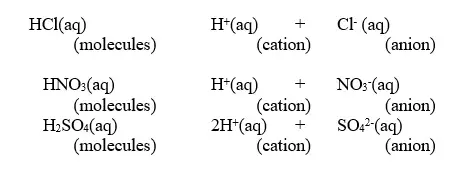
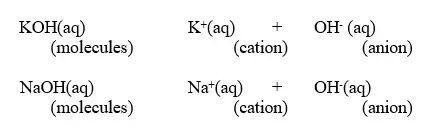

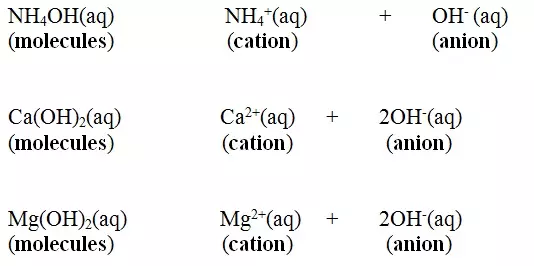
8. The concentration of an acid/base/alkali is based on the number of moles of acid/bases dissolved in a decimeter(litre)of the solution.
An acid/base/alkali with more acid/base/alkali in a decimeter(litre) of solution is said to be concentrated while that with less is said to be dilute.
9. (a) (i)strong acids have pH 1/2/3 while weak acids have high pH 4/5/6.
(ii) a neutral solution have pH 7.(iii) strong alkalis/bases have pH 12/13/14 while weak bases/alkalis have pH 11/10 /9 / 8.
(b) pH is a measure of H+(aq) concentration in a solution.
The higher the H+(aq)ions concentration ;
-
The higher the acidity
The lower the pH
The lower the concentration of OH-(aq)
The lower the alkalinity
- Beyond pH 7,the concentration of the OH-(aq) increases as the H+(aq) ions decreases.
10. (a) When acids /bases dissolve in water, the ions present in the solution conduct electricity.
The more the dissociation the higher the yield of ions and the greater the electrical conductivity of the solution.
A compound that conducts electricity in an electrolyte and thus a compound showing high electrical conductivity is a strong electrolyte while a compound showing low electrical conductivity is a weak electrolyte.
(b) Practically, a bright light on a bulb ,a high voltage reading from a voltmeter high ammeter reading from an ammeter, a big deflection on a galvanometer is an indicator of strong electrolyte(acid/base) and the opposite for weak electrolytes(acids/base).
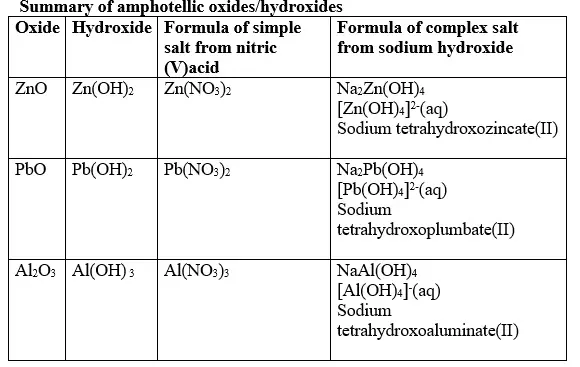
11. Some compounds exhibit/show both properties of acids and bases/alkalis.
- A substance that reacts with both acids and bases is said to be amphotellic.- The examples below show the amphotellic properties of:
(a) Zinc (II)oxide(ZnO) and Zinc hydroxide(Zn(OH)2)
(i)When ½ spatula full of Zinc(II)oxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium hydroxide hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.-
(i) When reacting with nitric(V)acid, the oxide shows basic properties by reacting with an acid to form a simple salt and water only.
Basic oxide + Acid → salt + water
Chemical equation
-
ZnO(s) + 2HNO3(aq) → Zn(NO3) 2 (aq) + H2O(l)
ZnO(s) + 2HCl(aq) → ZnCl2 (aq) + H2O(l)
ZnO(s) + H2SO4(aq) → ZnSO4 (aq) + H2O(l)
-
ZnO(s) + 2H+ (aq) → Zn2+ (aq) + H2O(l)
-
Basic oxide + Base/alkali + Water → Complex salt
Chemical equation
1. When Zinc oxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxozincate(II) complex salt.
-
ZnO(s) + 2NaOH(aq) + H2O(l) → Na2Zn(OH)4(aq)
-
ZnO(s) + 2KOH(aq) + H2O(l) → K2Zn(OH)4(aq)
-
ZnO(s) + 2OH-(aq) + H2O(l) → 2[Zn(OH)4]2- (aq)
-
(i) When reacting with nitric(V)acid, the hydroxide shows basic properties. It reacts with an acid to form a simple salt and water only.
Basic hydroxide + Acid → salt + water
Chemical equation
-
Zn(OH)2 (s) + 2HNO3(aq) → Zn(NO3) 2 (aq) + 2H2O(l)
Zn(OH)2 (s) + 2HCl(aq) → ZnCl2 (aq) + 2H2O(l)
Zn(OH)2 (s) + H2SO4(aq) → ZnSO4 (aq) + 2H2O(l)
-
Zn(OH) 2 (s) + 2H+ (aq) → Zn2+ (aq) + 2H2O(l)
-
Basic hydroxide + Base/alkali → Complex salt
Chemical equation
1. When Zinc hydroxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxozincate(II) complex salt.
-
Zn(OH)2 (s) + 2NaOH(aq) → Na2Zn(OH)4(aq)
-
Zn(OH)2 (s) + 2KOH(aq) → K2Zn(OH)4(aq)
-
Zn(OH)2 (s) + 2OH-(aq) → 2[Zn(OH)4]2- (aq)
(b) Lead (II)oxide(PbO) and Lead(II) hydroxide (Pb(OH)2)
(i) When ½ spatula full of Lead(II)oxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium hydroxide hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.(i) When reacting with nitric(V)acid, the oxide shows basic properties by reacting with an acid to form a simple salt and water only. All other Lead salts are insoluble.
Chemical equation
-
PbO(s) + 2HNO3(aq) → Pb(NO3) 2 (aq) + H2O(l)
-
PbO(s) + 2H+ (aq) → Pb2+ (aq) + H2O(l)
Chemical equation
1. When Lead(II) oxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxoplumbate(II) complex salt.
-
PbO(s) + 2NaOH(aq) + H2O(l) → Na2Pb(OH)4(aq)
-
PbO(s) + 2KOH(aq) + H2O(l) → K2Pb(OH)4(aq)
-
PbO(s) + 2OH-(aq) + H2O(l) → 2[Pb(OH)4]2- (aq)
(i) when reacting with nitric(V)acid, the hydroxide shows basic properties. It reacts with the acid to form a simple salt and water only.
Chemical equation
-
Pb(OH)2 (s) + 2HNO3(aq) → Pb(NO3) 2 (aq) + 2H2O(l)
-
Pb(OH)2 (s) + 2H+ (aq) → Pb2+(aq) + 2H2O(l)
Chemical equation
1. When Lead(II) hydroxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxoplumbate(II) complex salt.
-
Pb(OH)2 (s) + 2NaOH(aq) → Na2Pb(OH)4(aq)
-
Pb(OH)2 (s) + 2KOH(aq) → K2Pb(OH)4(aq)
-
Pb(OH)2 (s) + 2OH-(aq) → 2[Pb(OH)4]2- (aq)
(c) Aluminium(III)oxide(Al2O3) and Aluminium(III)hydroxide(Al(OH)3)
(i)When ½ spatula full of Aluminium(III)oxide is placed in a boiling tube containing 10cm3 of either 2M nitric(V)acid or 2M sodium hydroxide hydroxide solution, it dissolves on both the acid and the alkali/base to form a colourless solution. i.e.(i) when reacting with nitric(V)acid, the oxide shows basic properties by reacting with an acid to form a simple salt and water only.
Chemical equation
-
Al2O3 (s) + 6HNO3(aq) → Al(NO3)3 (aq) + 3H2O(l)
Al2O3 (s) + 6HCl(aq) → AlCl3 (aq) + 3H2O(l)
Al2O3 (s) + 3H2SO4(aq) → Al2(SO4)3 (aq) + 3H2O(l)
-
Al2O3 (s) + 3H+ (aq) → Al3+ (aq) + 3H2O(l)
Chemical equation
1. When Aluminium(III) oxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxoaluminate(III) complex salt.
-
Al2O3 (s) + 2NaOH(aq) + 3H2O(l) → 2NaAl(OH)4(aq)
-
Al2O3 (s) + 2KOH(aq) + 3H2O(l) → 2NaAl(OH)4(aq)
-
Al2O3 (s) + 2OH-(aq) + 3H2O(l) → 2[Al(OH)4]- (aq)
(i) when reacting with nitric(V)acid, the hydroxide shows basic properties. It reacts with the acid to form a simple salt and water only.
Chemical equation
Al(OH)3 (s) + 3HNO3(aq) → Al(NO3)3 (aq) + 3H2O(l)
Al(OH)3 (s) + 3HCl(aq) → AlCl3 (aq) + 3H2O(l)
2Al(OH)3 (s) + 3H2SO4(aq) → Al2(SO4)3 (aq) + 3H2O(l)
Ionic equationAl(OH)3 (s) + 3HCl(aq) → AlCl3 (aq) + 3H2O(l)
2Al(OH)3 (s) + 3H2SO4(aq) → Al2(SO4)3 (aq) + 3H2O(l)
-
Al(OH)3 (s) + 3H+ (aq) → Al3+ (aq) + 3H2O(l)
Chemical equation
1. When aluminium(III) hydroxide is reacted with sodium hydroxide the complex salt is sodium tetrahydroxoaluminate(III) complex salt.
-
Al(OH)3 (s) + NaOH(aq) → NaAl(OH)4(aq)
-
Al(OH)3 (s) + KOH(aq) → KAl(OH)4(aq)
-
Al(OH)3 (s) + OH-(aq) → [Al(OH)4]- (aq)
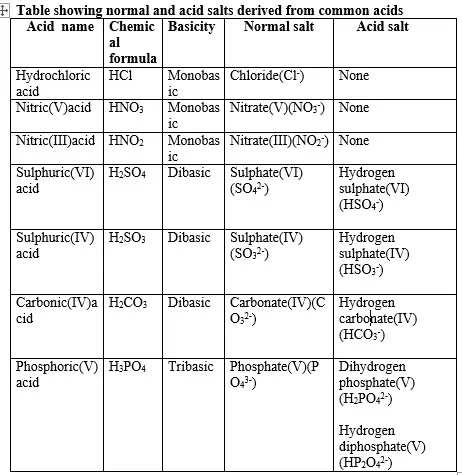
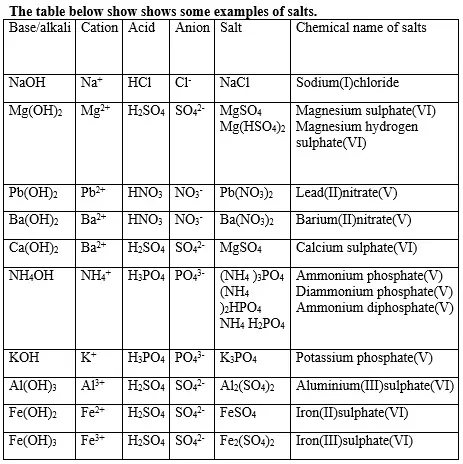
12. (a) A salt is an ionic compound formed when the cation from a base combine with the anion derived from an acid.
A salt is therefore formed when the hydrogen ions in an acid are replaced wholly/fully or partially/partly ,directly or indirectly by a metal or ammonium radical.(b) The number of ionizable/replaceable hydrogen in an acid is called basicity of an acid.
Some acids are therefore:
-
(i)Monobasic acids generally denoted HX e.g. HCl, HNO3,HCOOH,CH3COOH.
(ii)Dibasic acids ; generally denoted H2X e.g. H2SO4, H2SO3, H2CO3,HOOCOOH.
(iii)Tribasic acids ; generally denoted H4X e.g. H3PO4.
-
(i)A normal salt is formed when all the ionizable /replaceable hydrogen in an acid is replaced by a metal or metallic /ammonium radical.
(ii)An acid salt is formed when part/portion the ionizable /replaceable hydrogen in an acid is replaced by a metal or metallic /ammonium radical.
(d) Some salts undergo hygroscopy, deliquescence and efflorescence.
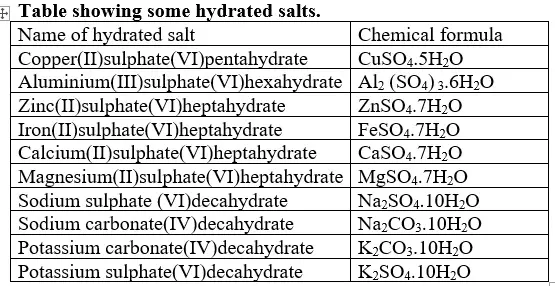
(i) Hygroscopic salts /compounds are those that absorb water from the atmosphere but do not form a solution.
Some salts which are hygroscopic include anhydrous copper(II)sulphate(VI), anhydrous cobalt(II)chloride, potassium nitrate(V) common table salt.
(ii)Deliquescent salts /compounds are those that absorb water from the atmosphere and form a solution.
Some salts which are deliquescent include: Sodium nitrate(V),Calcium chloride, Sodium hydroxide, Iron(II)chloride, Magnesium chloride.
(iii)Efflorescent salts/compounds are those that lose their water of crystallization to the atmosphere.
Some salts which effloresces include: sodium carbonate decahydrate, Iron(II)sulphate(VI)heptahydrate, sodium sulphate (VI)decahydrate.
(e)Some salts contain water of crystallization.They are hydrated.Others do not contain water of crystallization. They are anhydrous.
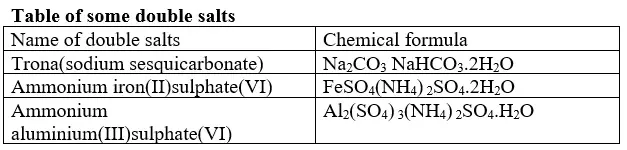
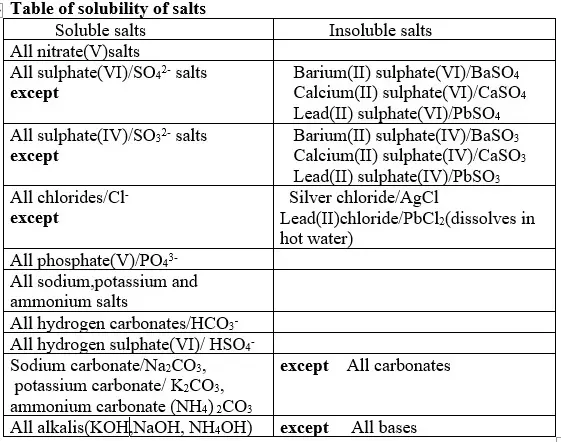
13. Salts can be prepared in a school laboratory by a method that uses its solubility in water.
(a) Soluble salts may be prepared by using any of the following methods:(i) Direct displacement/reaction of a metal with an acid.
- Excess of the metal must be used to ensure all the acid has reacted.- When effervescence/bubbling /fizzing has stopped ,excess metal is filtered.
- The filtrate is heated to concentrate then allowed to crystallize.
- Washing with distilled water then drying between filter papers produces a sample crystal of the salt. i.e.
-
M(s) + H2X → MX(aq) + H2(g)
Mg(s) + H2SO4(aq) → MgSO4 (aq) + H2(g)
Zn(s) + H2SO4(aq) → ZnSO4 (aq) + H2(g)
Pb(s) + 2HNO3(aq) → Pb(NO3) 2(aq) + H2(g)
Ca(s) + 2HNO3(aq) → Ca(NO3) 2(aq) + H2(g)
Mg(s) + 2HNO3(aq) → Mg(NO3) 2(aq) + H2(g)
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Zn(s) + H2SO4(aq) → ZnSO4 (aq) + H2(g)
Pb(s) + 2HNO3(aq) → Pb(NO3) 2(aq) + H2(g)
Ca(s) + 2HNO3(aq) → Ca(NO3) 2(aq) + H2(g)
Mg(s) + 2HNO3(aq) → Mg(NO3) 2(aq) + H2(g)
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
(ii) Reaction of an insoluble base with an acid
- By adding an insoluble base (oxide/hydroxide )to a dilute acid until no more dissolves, in the acid,a salt and water are formed.- Excess of the base is filtered off.
- The filtrate is heated to concentrate ,allowed to crystallize then washed with distilled water before drying between filter papers e.g.
PbO(s) + 2HNO3(aq) → Pb(NO3) 2(aq) + H2O (l)
Pb(OH)2(s) + 2HNO3(aq) → Pb(NO3) 2(aq) + 2H2O (l)
CaO (s) + 2HNO3(aq) → Ca(NO3) 2(aq) + H2O (l)
MgO (s) + 2HNO3(aq) → Mg(NO3) 2(aq) + H2O (l)
MgO (s) + 2HCl(aq) → MgCl2(aq) + H2O (l)
ZnO (s) + 2HCl(aq) → ZnCl2(aq) + H2O (l)
Zn(OH)2(s) + 2HNO3(aq) → Zn(NO3) 2(aq) + 2H2O (l)
CuO (s) + 2HCl(aq) → CuCl 2(aq) + H2O (l)
CuO (s) + H2SO4(aq) → CuSO4(aq) + H2O (l)
Ag2O(s) + 2HNO3(aq) → 2AgNO3(aq) + H2O (l)
Na2O(s) + 2HNO3(aq) → 2NaNO3(aq) + H2O (l)
Pb(OH)2(s) + 2HNO3(aq) → Pb(NO3) 2(aq) + 2H2O (l)
CaO (s) + 2HNO3(aq) → Ca(NO3) 2(aq) + H2O (l)
MgO (s) + 2HNO3(aq) → Mg(NO3) 2(aq) + H2O (l)
MgO (s) + 2HCl(aq) → MgCl2(aq) + H2O (l)
ZnO (s) + 2HCl(aq) → ZnCl2(aq) + H2O (l)
Zn(OH)2(s) + 2HNO3(aq) → Zn(NO3) 2(aq) + 2H2O (l)
CuO (s) + 2HCl(aq) → CuCl 2(aq) + H2O (l)
CuO (s) + H2SO4(aq) → CuSO4(aq) + H2O (l)
Ag2O(s) + 2HNO3(aq) → 2AgNO3(aq) + H2O (l)
Na2O(s) + 2HNO3(aq) → 2NaNO3(aq) + H2O (l)
(iii) Reaction of insoluble /soluble carbonate /hydrogen carbonate with an acid.
- By adding an excess of a soluble /insoluble carbonate or hydrogen carbonate to a dilute acid, effervescence /fizzing/bubbling out of carbon(IV)oxide gas shows the reaction is taking place.- When effervescence /fizzing/bubbling out of the gas is over, excess of the insoluble carbonate is filtered off.
- The filtrate is heated to concentrate ,allowed to crystallize then washed with distilled water before drying between filter paper papers e.g.
PbCO3 (s) + 2HNO3(aq) → Pb(NO3) 2(aq) + H2O (l)+ CO2(g)
ZnCO3 (s) + 2HNO3(aq) → Zn(NO3) 2(aq) + H2O (l)+ CO2(g)
CaCO3 (s) + 2HNO3(aq) → Ca(NO3) 2(aq) + H2O (l)+ CO2(g)
MgCO3 (s) + H2SO4(aq) → MgSO4(aq) + H2O (l)+ CO2(g)
CuCO3 (s) + H2SO4(aq) → CuSO4(aq) + H2O (l) + CO2(g)
Ag2CO3 (s) + 2HNO3(aq) → 2AgNO3(aq) + H2O (l) + CO2(g)
Na2CO3 (s) + 2HNO3(aq) → 2NaNO3(aq) + H2O (l) + CO2(g)
K2CO3 (s) + 2HCl(aq) → 2KCl(aq) + H2O (l) + CO2(g)
NaHCO3 (s) + HNO3(aq) → NaNO3(aq) + H2O (l) + CO2(g)
KHCO3 (s) + HCl(aq) → KCl(aq) + H2O (l) + CO2(g)
ZnCO3 (s) + 2HNO3(aq) → Zn(NO3) 2(aq) + H2O (l)+ CO2(g)
CaCO3 (s) + 2HNO3(aq) → Ca(NO3) 2(aq) + H2O (l)+ CO2(g)
MgCO3 (s) + H2SO4(aq) → MgSO4(aq) + H2O (l)+ CO2(g)
CuCO3 (s) + H2SO4(aq) → CuSO4(aq) + H2O (l) + CO2(g)
Ag2CO3 (s) + 2HNO3(aq) → 2AgNO3(aq) + H2O (l) + CO2(g)
Na2CO3 (s) + 2HNO3(aq) → 2NaNO3(aq) + H2O (l) + CO2(g)
K2CO3 (s) + 2HCl(aq) → 2KCl(aq) + H2O (l) + CO2(g)
NaHCO3 (s) + HNO3(aq) → NaNO3(aq) + H2O (l) + CO2(g)
KHCO3 (s) + HCl(aq) → KCl(aq) + H2O (l) + CO2(g)
(iv) Neutralization/reaction of soluble base/alkali with dilute acid
By adding an acid to a burette into a known volume of an alkali with 2-3 drops of an indicator, the colour of the indicator changes when the acid has completely reacted with an alkali at the end point.The procedure is then repeated without the indicator .The solution mixture is then heated to concentrate , allowed to crystallize ,washed with distilled water before drying with filter papers. e.g.
-
NaOH (aq) + HNO3(aq) → NaNO3(aq) + H2O (l)
KOH (aq) + HNO3(aq) → KNO3(aq) + H2O (l)
KOH (aq) + HCl(aq) → KCl(aq) + H2O (l)
2KOH (aq) + H2SO4(aq) → K2SO4(aq) + 2H2O (l)
NH4OH (aq) + HNO3(aq) → NH4NO3(aq) + H2O (l)
(iv) Direct synthesis/combination.
When a metal burn in a gas jar containing a non metal, the two directly combine to form a salt. e.g.-
2Na(s) + Cl2(g) → 2NaCl(s)
2K(s) + Cl2(g) → 2KCl(s)
Mg(s) + Cl2(g) → MgCl2 (s)
Ca(s) + Cl2(g) → CaCl2 (s)
- Care should be taken to avoid water/moisture into the reaction flask during their preparation.
- Such salts include aluminium(III)chloride(AlCl3) and iron (III)chloride(FeCl3).
1. Heated aluminium foil reacts with chlorine to form aluminium(III)chloride that sublimes away from the source of heating then deposited as solid again.
-
2Al(s) + 3Cl2(g) → 2AlCl3 (s/g)
-
AlCl3(s)+ 3H2O(g) → Al(OH)3 (aq) + 3HCl(g)
-
2Fe(s) + 3Cl2(g) → 2FeCl3 (s/g)
-
FeCl3(s)+ 3H2O(g) → Fe(OH)3 (aq) + 3HCl(g)
- This is called double decomposition or precipitation.
- The mixture is filtered and the residue is washed with distilled water then dried.
-
CuSO4(aq) + Na2CO3 (aq) → CuCO3 (s) + Na2SO4(aq)
BaCl2(aq) + K2SO4 (aq) → BaSO4 (s) + 2KCl (aq)
Pb(NO3)2(aq) + K2SO4 (aq) → PbSO4 (s) + 2KNO3 (aq)
2AgNO3(aq) + MgCl2 (aq) → 2AgCl(s) + Mg(NO3)2 (aq)
Pb(NO3)2(aq) + (NH4)2SO4 (aq) → PbSO4 (s) + 2NH4NO3(aq)
BaCl2(aq) + K2SO3 (aq) → BaSO4 (s) + 2KCl (aq)
14. Salts may lose their water of crystallization , decompose ,melt or sublime on heating on a Bunsen burner flame.
- The following shows the behavior of some salts on heating gently /or strongly in a laboratory school burner:(a) Effect of heat on Chlorides.
All chlorides have very high melting and boiling points and therefore are not affected by laboratory heating except ammonium chloride. Ammonium chloride sublimes on gentle heating. It dissociate into the constituent ammonia and hydrogen chloride gases on strong heating.-
NH4Cl(s) [(sublimation)] NH4Cl(g) : NH3(g) + HCl(g) [(dissociation)]
(b) Effect of heat on nitrate .
(i) Potassium nitrate(V)/KNO3 and sodium nitrate(V)/NaNO3 decompose on heating to form Potassium nitrate(III)/KNO2 and sodium nitrate(III)/NaNO2 and producing Oxygen gas in each case. -
2KNO3 (s) → 2KNO2(s) + O2(g)
2NaNO3 (s) → 2NaNO2(s) + O2(g)
2Mg(NO3)2(s) → 2MgO(s) + 4NO2(g) + O2(g)
2Zn(NO3)2(s) → 2ZnO(s) + 4NO2(g) + O2(g)
2Pb(NO3)2(s) → 2PbO(s) + 4NO2(g) + O2(g)
2Cu(NO3)2(s) → 2CuO(s) + 4NO2(g) + O2(g)
2Fe(NO3)2(s) → 2FeO(s) + 4NO2(g) + O2(g)
-
2AgNO3(s) → 2Ag (s) + 2NO2(g) + O2(g)
2Hg(NO3)2 (s) → 2Hg (s) + 4NO2(g) + O2(g)
-
NH4NO3(s) → N2O (g) + H2O(l)
NH4NO2(s) → N2(g) + H2O(l)
(c) Effect of heat on Sulphate
Only Iron(II)sulphate(VI), Iron(III)sulphate(VI) and copper(II)sulphate(VI) decompose on heating. They form the oxide, and produce highly acidic fumes of acidic sulphur(IV)oxide gas.-
2FeSO4 (s) → Fe2O3(s) + SO3(g) + SO2(g)
Fe2(SO4) 3(s) → Fe2O3(s) + SO3(g)
CuSO4 (s) → CuO(s) + SO3(g)
(d) Effect of heat on carbonates(IV) and hydrogen carbonate(IV).
(i)Sodium carbonate(IV)and potassium carbonate(IV)do not decompose on heating.(ii)Heavy metal nitrate(IV)salts decompose on heating to form the oxide and produce carbon(IV)oxide gas. Carbon (IV)oxide gas forms a white precipitate when bubbled in lime water. The white precipitate dissolves if the gas is in excess. e.g.
-
CuCO3 (s) → CuO(s) + CO2(g)
CaCO3 (s) → CaO(s) + CO2(g)
PbCO3 (s) → PbO(s) + CO2(g)
FeCO3 (s) → FeO(s) + CO2(g)
ZnCO3 (s) → ZnO(s) + CO2(g)
-
2NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(l)
2KHCO3(s) → K2CO3(s) + CO2(g) + H2O(l)
-
Ca(HCO3)2(aq) → CaCO3(s) + CO2(g) + H2O(l)
Mg(HCO3)2(aq) → MgCO3(s) + CO2(g) + H2O(l)
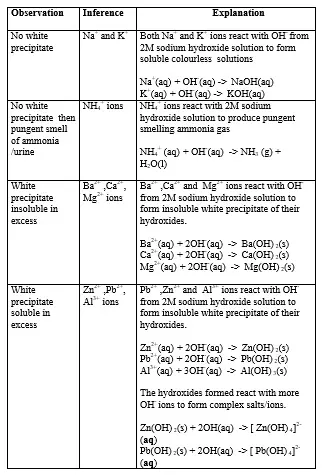
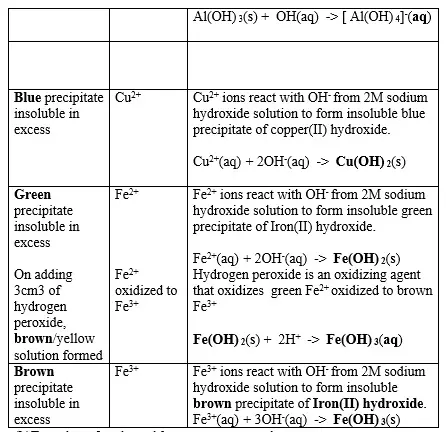
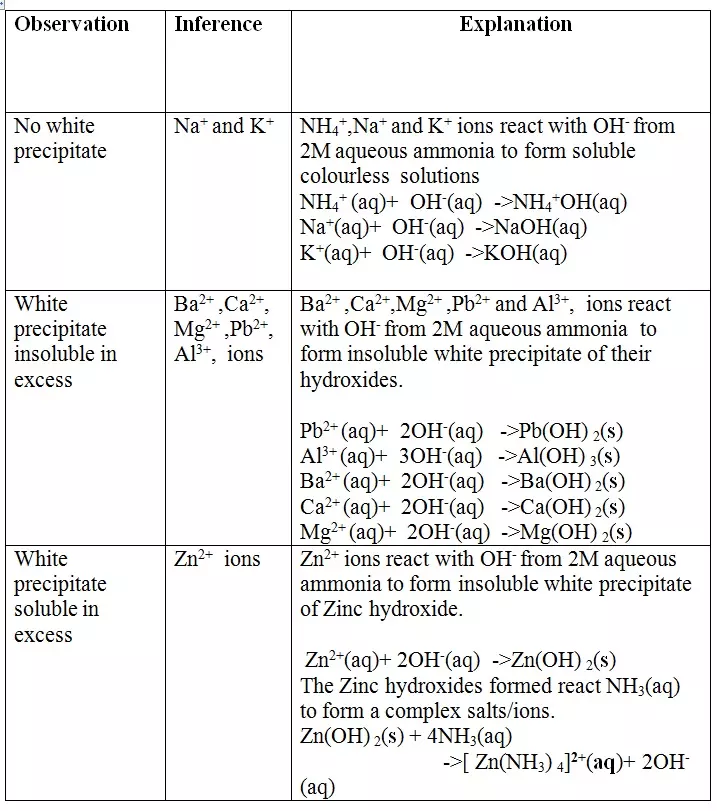
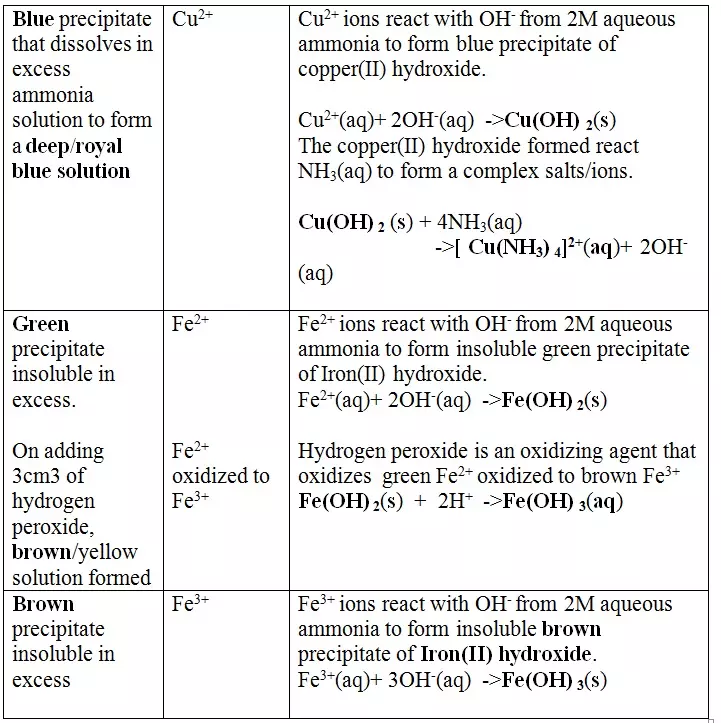
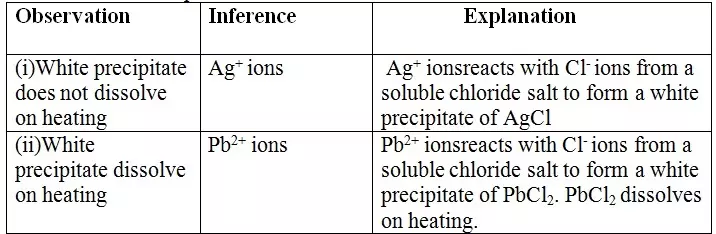
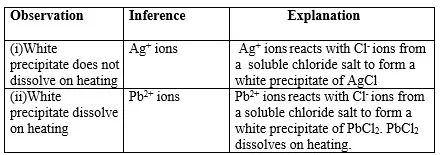
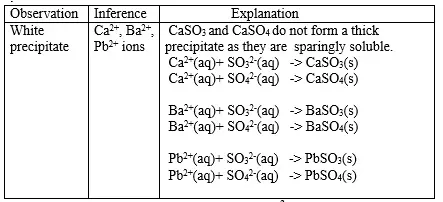
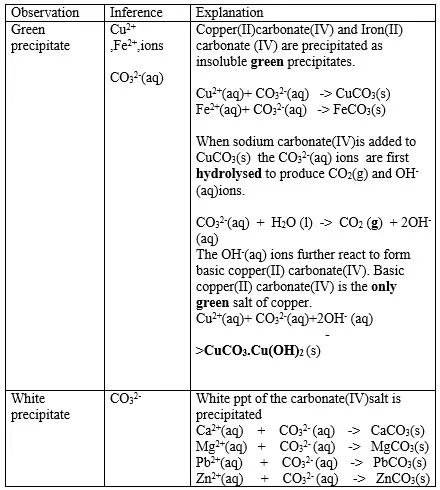
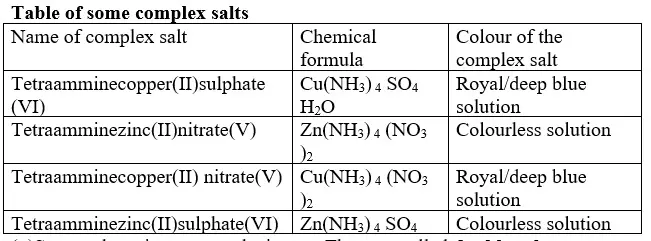
15. Salts contain cation(positively charged ion) and anions(negatively charged ion).When dissolved in polar solvents/water.
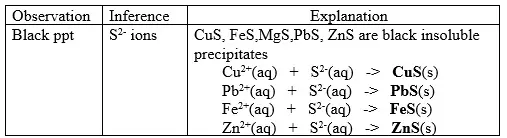
- The cation and anion in a salt is determined/known usually by precipitation of the salt using a precipitating reagent.- The colour of the precipitate is a basis of qualitative analysis of a compound.
16. Qualitative analysis is the process of identifying an unknown compound /salt by identifying the unique qualities of the salt/compound.
It involves some of the following processes.