Question
Study the information below and answer the following questions. A mixture contains three solids A, B, and C. The solubility of these solids in different liquids is as shown below:
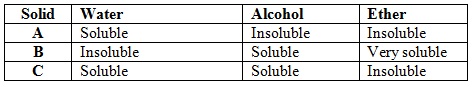
Explain how you will obtain sample C from the mixture

Explain how you will obtain sample C from the mixture
Answer
a) Add ether to the mixture. Stir and filter
b) Add alcohol to the residue, stir and filter
c) Evaporate to filtrate to obtain C
b) Add alcohol to the residue, stir and filter
c) Evaporate to filtrate to obtain C

