Question
The flow chart below shows the preparation of carbon (II) oxide and its reaction.
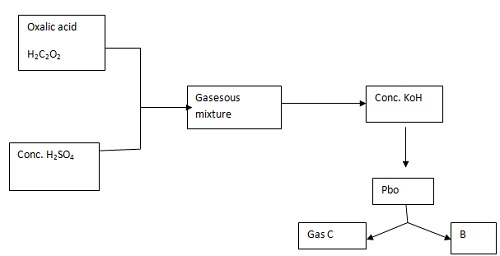
a. Name the type of reaction that takes place between H2C2O2 and conc. H2SO4.
b. Write an equation for the production of B and C.
c. State two uses of carbon (II) oxide.

a. Name the type of reaction that takes place between H2C2O2 and conc. H2SO4.
b. Write an equation for the production of B and C.
c. State two uses of carbon (II) oxide.
Answer
a. Dehydration

c.
-- Water gas - A fuel
-- Reducing agent in the extraction of metals

c.
-- Water gas - A fuel
-- Reducing agent in the extraction of metals

