Question
The set up below was used to collect a dry sample of a gas
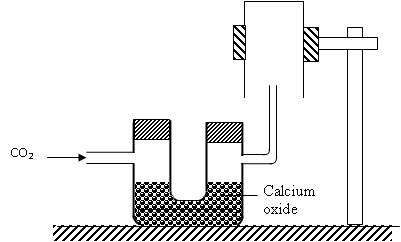
Give two reasons why the set up cannot be used to collect carbon (IV) oxide.

Give two reasons why the set up cannot be used to collect carbon (IV) oxide.
Answer
i) The Co2 will react with the CaO as it is basic.
ii) Because of wrong method of collection. Co2 is denser than air
ii) Because of wrong method of collection. Co2 is denser than air

