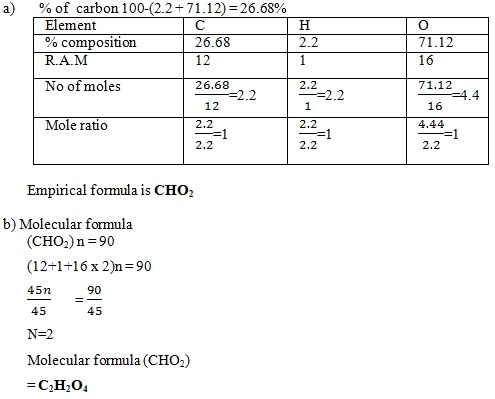
Question
A compound of carbon, hydrogen and oxygen contains 71.12% oxygen, 2.2% hydrogen and rest carbon. If it has a relative molecular mass of 90.
a) Determine the empirical formula of the compound C = 12, 0 = 6 H = 1
b) Determine the molecular formula of the compound
a) Determine the empirical formula of the compound C = 12, 0 = 6 H = 1
b) Determine the molecular formula of the compound
Answer