Pure water does not exist in nature but naturally in varying degree of purity.
The main sources of water include rain, springs, borehole, lakes, seas and oceans
Main Uses of Water
- Drinking by animals and plants.
- Washing clothes.
- Bleaching and dyeing.
- Generating hydroelectric power.
- Cooling industrial processes.
NOTEs:
- Water dissolves many substances/solutes.
- It is therefore called universal solvent.
- It contains about 35% dissolved Oxygen which support aquatic fauna and flora.
- Water naturally exists in three phases/states solid ice, liquid water and gaseous water vapour.
- The three states of water are naturally inter-convertible in the water cycle as shown below
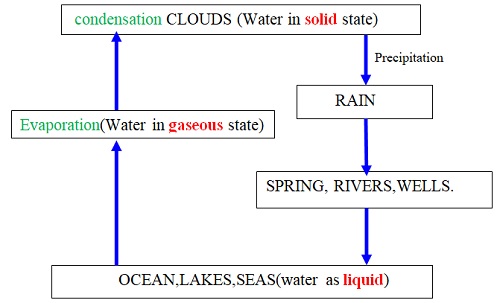
Explanation of the Water Cycle
Liquid water in land, lakes, seas and oceans use the solar/sun energy to evaporate/vapourize to form water vapour/gas. Solar/sun energy is also used during transpiration by plants and respiration by animals. During evaporation, the water vapour rises up the earth’s surface. Temperatures decrease with height above the earth surface increase. Water vapour therefore cools as it rises up. At a height where it is cold enough to below 373Kelvin/100oC Water vapour looses enough energy to form tiny droplets of liquid. The process by which a gas/water vapour changes to a liquid is called condensation/liquification. On further cooling, the liquid looses more energy to form ice/solid. The process by which a liquid/water changes to a ice/solid is called freezing/solidification. Minute/tiny ice/solid particles float in the atmosphere and coalesce/join together to form clouds. When the clouds become too heavy they fall to the earth’s surface as rain/snow as the temperature increase with the fall.Interconversion of the Three Phases of Matter
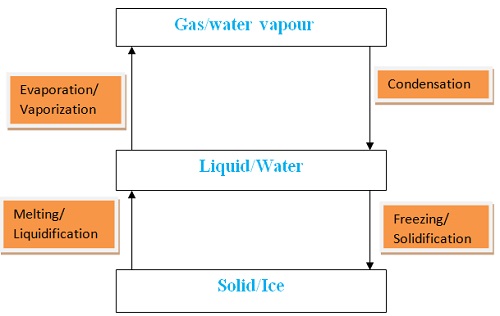
- It has fixed/constant/sharp freezing point/melting point of 273K/0oC
- It has fixed/constant/sharp boiling point of 373K/100oC at sea level/1 atmosphere pressure
- It has fixed density of 1gcm-3
Test for Presence of Water using anhydrous copper (II) sulphate
The procedure for the experiment is conducted as follows;- Put about 2g of anhydrous copper (II) sulphate crystals into a clean test tube
- Add three drops of tap water.
- Repeat the procedure using distilled water.
NOTES:
- The colour changes from white to blue
- Anhydrous copper (II) sulphate is a white substance
- Anhydrous copper (II) sulphate gains/reacts with water to form hydrated copper (II) sulphate, which is blue
- Hydrated copper (II) sulphate contains water of crystallization.
- The confirmatory test of water presence is the change of white anhydrous copper (II) sulphate to blue hydrated copper (II) sulphate
Anhydrous copper (II) sulphate (white) + Water
 Hydrated copper (II) sulphate (blue)
Hydrated copper (II) sulphate (blue)CuSO4(s) + 5H2O (l)
 CuSO4.5H2O(s)
CuSO4.5H2O(s)Test for Presence of Water using anhydrous cobalt (II) chloride
The procedure for the experiment is conducted as follows;- Put about 5cm3 of water into a clean test tube.
- Dip a dry anhydrous cobalt (II) chloride paper into the test tube
- Repeat the procedure using distilled water.
NOTES:
- The color changes from blue to pink
- Anhydrous cobalt (II) chloride is blue
- Anhydrous cobalt (II) chloride gains/reacts with water to form hydrated cobalt (II) chloride, which is pink
- Hydrated cobalt (II) chloride contains water of crystallization.
- This is another confirmatory test of water where blue anhydrous cobalt (II) chloride change to pink hydrated cobalt (II) chloride
Anhydrous cobalt (II) chloride (blue) + Water
 Hydrated cobalt (II) chloride (pink)
Hydrated cobalt (II) chloride (pink)CoCl2 (s) + 5H2O (l)
 CoCl2.5H2O(s)
CoCl2.5H2O(s)Burning a Candle in Air
The procedure for the experiment is conducted as follows;- Put about 2g of anhydrous copper (II) sulphate (VI) crystals in a boiling tube.
- Put about 5cm3 of lime water in a boiling tube.
- Light a small candle stick and place it below an inverted thistle/filter funnel
- Collect the products of the burning candle by setting the apparatus as below
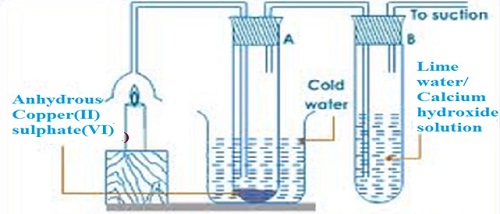
- The sanction pump pulls the products of burning into the inverted funnel.
- Colour of anhydrous copper (II) sulphate (VI) changes from white to blue.
- A white precipitate is formed in the lime water/calcium hydroxide.
- When a candle burn it forms a water and carbon (IV) oxide.
- Water turns anhydrous copper (II) sulphate (VI) changes from white to blue.
- Carbon (IV) oxide gas forms white precipitate when bubbled in lime water/calcium hydroxide.
- Hydrogen in the wax burn to form water.
- Carbon in the wax burn to form carbon (IV) oxide - The candle before burning therefore contained only Carbon and Hydrogen only.
- A compound made up of hydrogen and carbon is called Hydrocarbon.
- A candle is a hydrocarbon.
- Hydrocarbons burn in air to form water and carbon (IV) oxide gas.
Water Pollution
This occurs when undesirable substances are added into the water.There are different sources of water pollution as follows;
Causes of Water Pollution
(i) Industrial chemicals being disposed into water bodies like rivers, lakes and oceans.(ii) Discharging untreated /raw sewage into water bodies.
(iii) Leaching of insecticides/herbicides form agricultural activities into water bodies.
(iv) Discharging non-biodegradable detergents after domestic and industrial use into water bodies.
(v) Petroleum oil spilling by ships and oil refineries
(vi) Toxic/poisonous gases from industries dissolving in rain.
(vii) Acidic gases from industries dissolving in rain to form “acid rain”
(viii) Discharging hot water into water bodies. This reduces the quantity of dissolved Oxygen in the water killing the aquatic fauna and flora.
Prevention of Water Pollution
It is important to reduce water pollution, which can be done by:(i) Reducing the use of agricultural fertilizers and chemicals in agricultural activities.
(ii) Use of biological control method instead of insecticides and herbicides
(iii) Using biodegradable detergents




