Question
Use the information in the table below and answer the question that follows
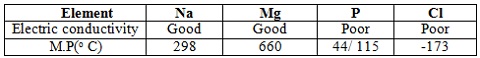
a. Explain why both sodium and magnesium conduct electricity while phosphorus and chlorine do not
b. Suggest a reason why phosphorus has been assigned two melting points values

a. Explain why both sodium and magnesium conduct electricity while phosphorus and chlorine do not
b. Suggest a reason why phosphorus has been assigned two melting points values
Answer
(a) Mg and Na has delocalised electrons in their metallic structure while Cl2 exist as a molecule, has no delocalised electrons.
(b) It has allotropes.
(b) It has allotropes.

