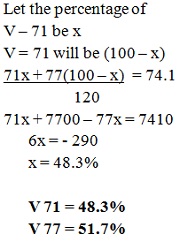Question
Element V exist as two isotopes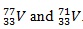 .
.
The relative atomic mass of a normal sample of V is 74.1. Deduce the relative percentage abundance of each Isotope.
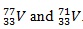 .
.The relative atomic mass of a normal sample of V is 74.1. Deduce the relative percentage abundance of each Isotope.
Answer