Question
The setup in the diagram below was used to study the reaction between magnesium ribbon and steam. Study it and answer the question that follows
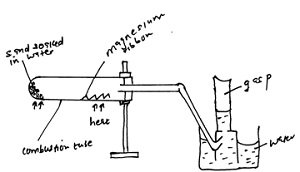
i. Identify gas P
ii. Explain why it is possible to collect gas P by the method shown
iii. Write the chemical equation for the reaction that took place in the combustion tube

i. Identify gas P
ii. Explain why it is possible to collect gas P by the method shown
iii. Write the chemical equation for the reaction that took place in the combustion tube
Answer
i) Hydrogen gas
ii) Slightly soluble in water
iii) Mg(s) + H2O(g) → MgO(s) + H2(g)
ii) Slightly soluble in water
iii) Mg(s) + H2O(g) → MgO(s) + H2(g)

