Question
A student left some crushed fruit mixture with water for some days. He found the mixture had fermented. He concluded that the mixture was contaminated with water and ethanol with boiling point of 100°C and 78°C respectively. The set-up of apparatus below are used to separate the mixture.
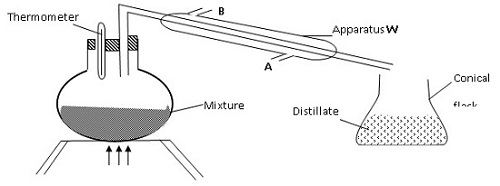
(i) Name the piece of apparatus labelled W
(ii) What is the purpose of the thermometer in the set-up?
iii) At which end of the apparatus W should tap water be connected?
(iv) Which liquid was collected as the first distillate? Explain
(v) What is the name given to the above method of separating mixture?
(vi) State two applications of the above method of separating mixtures
(vi) What properties of the mixture makes it possible for the component to be separated by the above methods?

(i) Name the piece of apparatus labelled W
(ii) What is the purpose of the thermometer in the set-up?
iii) At which end of the apparatus W should tap water be connected?
(iv) Which liquid was collected as the first distillate? Explain
(v) What is the name given to the above method of separating mixture?
(vi) State two applications of the above method of separating mixtures
(vi) What properties of the mixture makes it possible for the component to be separated by the above methods?
Answer
(i) Condenser
(ii) To indicate when a liquid is boiling, a thermometer reads a constant temperature
(iii) A
(iv) Ethanol
Reason:- It has a lower boiling of 78°C compared to water with a boiling point of 100°C
or
- The liquid with the lower boiling point boils first and its vapours are condensed and the condenser to be collected as the first distillate
(v) Fractional distillation
(vi) - To separate components of crude oil
-To isolate O2 and N2 from air
-To manufacture spirits
(vii)-They are immiscible liquids
-They have different but close boiling points
(ii) To indicate when a liquid is boiling, a thermometer reads a constant temperature
(iii) A
(iv) Ethanol
Reason:- It has a lower boiling of 78°C compared to water with a boiling point of 100°C
or
- The liquid with the lower boiling point boils first and its vapours are condensed and the condenser to be collected as the first distillate
(v) Fractional distillation
(vi) - To separate components of crude oil
-To isolate O2 and N2 from air
-To manufacture spirits
(vii)-They are immiscible liquids
-They have different but close boiling points

