Question
Majaliwa, a form one student at Kanga High School wanted to separate and obtain iodine and sodium chloride (common table salt) from a mixture of the two. he set the experimental set up shown below.
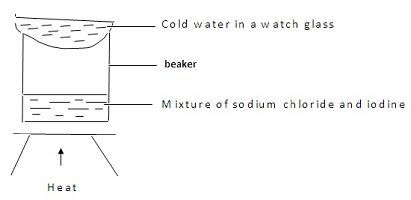
(a). the mixture was heated for some time and left to cool. On cooling, shiny black crystals and white crystals were observed on the surface of the watch glass and in the beaker respectively. Name:
I. Shiny black crystals
II. White crystals.
(b). What was the purpose of the cold water in the watch glass?
(c). Explain how the shiny black crystals on the surface of the watch glass is formed.
(d). What property of iodine makes it be collected on the watch glass as shown?
(e). Explain why it is possible to separate a mixture of iodine and sodium chloride.(1mk)

(a). the mixture was heated for some time and left to cool. On cooling, shiny black crystals and white crystals were observed on the surface of the watch glass and in the beaker respectively. Name:
I. Shiny black crystals
II. White crystals.
(b). What was the purpose of the cold water in the watch glass?
(c). Explain how the shiny black crystals on the surface of the watch glass is formed.
(d). What property of iodine makes it be collected on the watch glass as shown?
(e). Explain why it is possible to separate a mixture of iodine and sodium chloride.(1mk)
Answer
(a)(i) - shiny-black crystals are iodine crystals
-white crystals are sodium chloride solid
(b)To cool and condense the iodine vapour to form iodine solid.
(c) When the mixture is heated, iodine sublimes into purple vapour . on reaching the surface of the watch glass , it is cooled and condensed into shiny black crystals of iodine .
(d) iodine sublimes when heated .
(e) iodine sublimes while sodium chloride does not.
-white crystals are sodium chloride solid
(b)To cool and condense the iodine vapour to form iodine solid.
(c) When the mixture is heated, iodine sublimes into purple vapour . on reaching the surface of the watch glass , it is cooled and condensed into shiny black crystals of iodine .
(d) iodine sublimes when heated .
(e) iodine sublimes while sodium chloride does not.

