Question
The apparatus shown below was set to prepare and collect hydrogen sulphide gas.
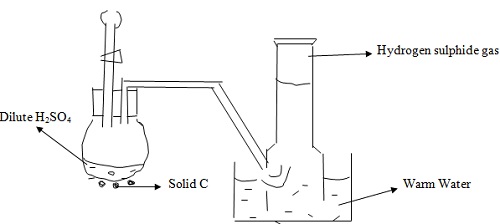
a. Name solid C.
b. Give a reason why warm water is used.
c. What observation would be made if hydrogen sulphate gas was bubbled into a solution of lead (II) nitrate?

a. Name solid C.
b. Give a reason why warm water is used.
c. What observation would be made if hydrogen sulphate gas was bubbled into a solution of lead (II) nitrate?
Answer
a. Iron (II) sulphide
b. Hydrogen sulphide is soluble in cold water
c. A black solid is formed
b. Hydrogen sulphide is soluble in cold water
c. A black solid is formed

