Question
In an experiment a test tube full of chlorine water was inverted in chlorine water as shown in the diagram below and then the set up left in the sunlight for one day.
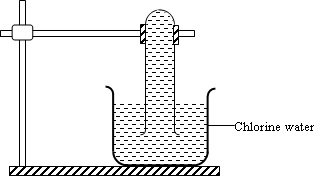
After one day, a gas was found to have collected in the test tube
a) Identify the gas
b) What will happen to the pH of the solution in the beaker after one day? Give a reason

After one day, a gas was found to have collected in the test tube
a) Identify the gas
b) What will happen to the pH of the solution in the beaker after one day? Give a reason
Answer
a) Oxygen gas
b) The pH of the solution goes down. This is because the concentration of Hydrogen ions increases (becomes more acidic)
b) The pH of the solution goes down. This is because the concentration of Hydrogen ions increases (becomes more acidic)

