Question
The apparatus below was set up to show the catalytic oxidation of ammonia. Study the diagram and answer the questions that follow
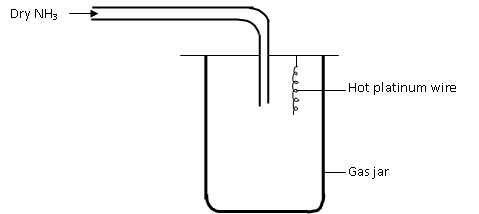
a) Write an equation for the reaction that takes place in the gas jar
b) Why is it necessary to have a hot platinum wire in the gas jar
c) After sometime brown fumes are observed in the gas jar. Explain on the observation.

a) Write an equation for the reaction that takes place in the gas jar
b) Why is it necessary to have a hot platinum wire in the gas jar
c) After sometime brown fumes are observed in the gas jar. Explain on the observation.
Answer
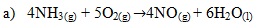
b) Acts as catalyst
c) The nitrogen (II) oxide formed is oxidized to brown fumes of nitrogen (IV) oxide.

