Question
a) The diagram below shows a set-up used by a student to find out what happens when Copper (II) sulphate crystals are heated.
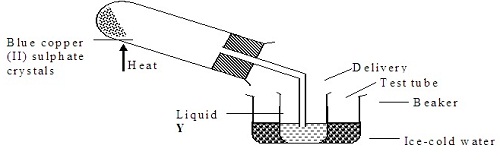
(i) State the observations made when the blue copper (II) sulphate crystals are heated.
(ii) Identify liquid Y and write an equation for its formation.
b) Pellets of sodium hydrogen and anhydrous Copper (II) sulphate were put in separate Petri- dishes and left in the open for two hours. Explain the observation in each Petri-dish.

(i) State the observations made when the blue copper (II) sulphate crystals are heated.
(ii) Identify liquid Y and write an equation for its formation.
b) Pellets of sodium hydrogen and anhydrous Copper (II) sulphate were put in separate Petri- dishes and left in the open for two hours. Explain the observation in each Petri-dish.
Answer
a) i) Colourless liquid is seen on the cooler parts of the test tube.
- Blue crystals change to a white powder
ii) Water which was originally water crystallization.
CuSO4 , 5H2O(s) = CuSO4(s) + 5H2O(l)
b) NaOH(s) absorbs water from the air and forms a solution. It is a deliquescent substance. Anhydrous CuSO4 absorbs water from air to form hydrated Copper (II) sulphate which is blue but no solution is formed it is hygroscopic
- Blue crystals change to a white powder
ii) Water which was originally water crystallization.
CuSO4 , 5H2O(s) = CuSO4(s) + 5H2O(l)
b) NaOH(s) absorbs water from the air and forms a solution. It is a deliquescent substance. Anhydrous CuSO4 absorbs water from air to form hydrated Copper (II) sulphate which is blue but no solution is formed it is hygroscopic

