Question
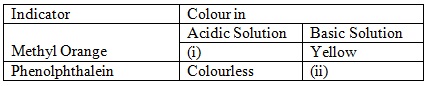
a) Complete the table below to show the colour of the given indicator in acidic and basic solutions.
b) How does the PH value of 0.1M potassium hydroxide solution compare with that of 0.1M aqueous ammonia? Explain.

b) How does the PH value of 0.1M potassium hydroxide solution compare with that of 0.1M aqueous ammonia? Explain.
Answer
(a)
(i) Red/Pink
(ii) Colourless/Pink
(b)The PH of 0.1M KOH is higher than of 0.1M aqueous ammonia KOH is strongly dissociated in solution.
(i) Red/Pink
(ii) Colourless/Pink
(b)The PH of 0.1M KOH is higher than of 0.1M aqueous ammonia KOH is strongly dissociated in solution.

