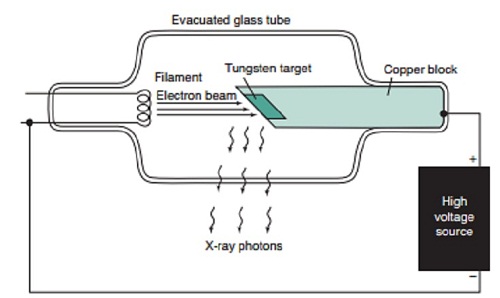
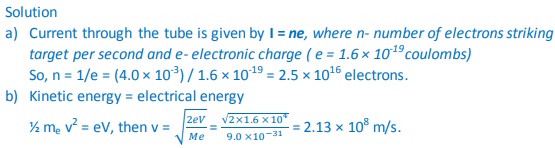- When the cathode is heated electrons are emitted by thermionic emission. They acquire electrical energy which can be expressed as E = e V.
- Once in motion the electrical energy is converted to kinetic energy, that is
eV =½ me v2.
- The energy of an electromagnetic wave can be calculated using the following equation:
Energy = h f, where h - Planck's constant, f – frequency of the wave.
- The highest frequency of the X-rays released after an electron hits the target is when the greatest kinetic energy is lost, that is
h f max = eV.
- Lower frequencies are released when the electrons make multiple collisions losing energy in stages, the minimum wavelength, λ min, of the emitted X-rays is given by;
(hc) / λ min = eV.
These expressions can be used to calculate the energy, frequencies and wavelengths of X-rays.
1. Determine the energy possessed by X-rays whose frequency is 4 × 1017 Hz.
Solution
E = h f => 6.63 × 10
-34 ×4 × 10
17 = 2.652 × 10
-16 J.
2. An x-ray tube operates at 60 kV and the current through it is 4.0 mA. Calculate the:
a) Number of electrons striking the target per second.
b) Speed of the electrons when they hit the target
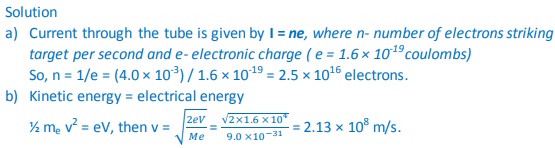
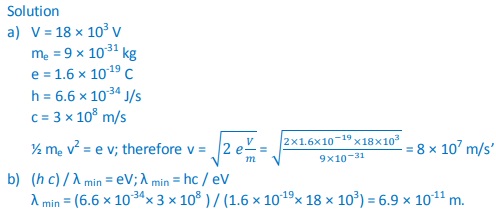
3. An 18 kV accelerating voltage is applied across an X-ray tube. Calculate:
a) The velocity of the fastest electron striking the target
b) The minimum wavelength in the continuous spectrum of X-rays produced. (mass of electron - 9 × 10-31 kg, charge on an electron-1.6 × 10-19 C, h- 6.6 × 10-34 J/s, c - 3 × 108 m/s)