Question 1
Define the following terms:
(i) Atomic Number
(ii) Mass Number
(iii) The Isotopes
Answer
(i) Atomic Number - Number of protons in the nucleus of an atom
(ii) Mass Number - Sum of proton and neutrons in the nucleus
(iii) The Isotopes - Element with same atomic no. but different mass number
(ii) Mass Number - Sum of proton and neutrons in the nucleus
(iii) The Isotopes - Element with same atomic no. but different mass number
Question 2
Oxygen is obtained on large scale by the fractional distillation of air as shown on the flow chart below.
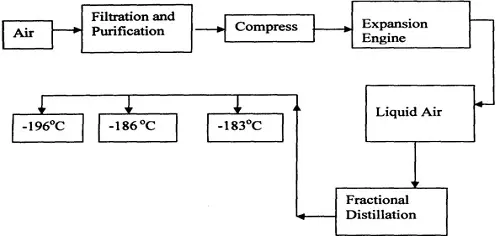
a) Explain why air is considered as a mixture
b) Identify the substance that is removed at the filtration stage
c) Explain why Carbon (IV) oxide and water are removed before liquefaction of air.
d) Identify the component that is collected at -186°C
Answer
a) Explain why air is considered as a mixture
- Various components can be separated using a physical means / method.
- Components in air are not in fixed proportions.
- It contains several gases which are not chemically combined
b) Identify the substance that is removed at the filtration stage
Dust particles
c) Explain why Carbon (IV) oxide and water are removed before liquefaction of air.
- They would readily solidify and block the pipes
d) Identify the component that is collected at -186°C
Argon
- Various components can be separated using a physical means / method.
- Components in air are not in fixed proportions.
- It contains several gases which are not chemically combined
b) Identify the substance that is removed at the filtration stage
Dust particles
c) Explain why Carbon (IV) oxide and water are removed before liquefaction of air.
- They would readily solidify and block the pipes
d) Identify the component that is collected at -186°C
Argon
Question 3
Study the table below and answer the questions that follow:-
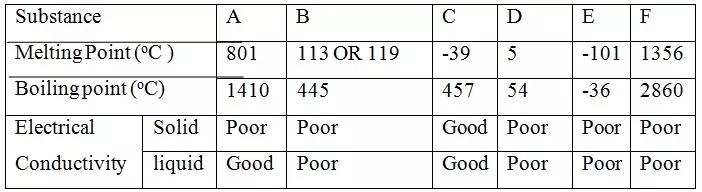
Identify with reasons the substances that:
(i) Have a metallic structure
(ii) Have a molecular structure
(iii) Substances A and C conduct electric current in the liquid state. State how the two substances differ as conductors of electric current
Answer
(i) Have a metallic structure
C - Good conductor of electricity in both solid and liquid state due to delocalized
(ii) Have a molecular structure
D or E - Are poor conducts in both solid / liquid state.
Have relatively low M.P and B.P due to molecular structure.
(iii) Substances A and C conduct electric current in the liquid state. State how the two substances differ as conductors of electric current
A – mobile/free ions
B – Delocalized electrons
C - Good conductor of electricity in both solid and liquid state due to delocalized
(ii) Have a molecular structure
D or E - Are poor conducts in both solid / liquid state.
Have relatively low M.P and B.P due to molecular structure.
(iii) Substances A and C conduct electric current in the liquid state. State how the two substances differ as conductors of electric current
A – mobile/free ions
B – Delocalized electrons
Question 4
Atoms of element X exists as:
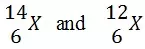
What name is given to the two types of atoms?
Answer
Isotopes - Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons
Question 5
Give two reasons why most laboratory apparatus are made of glass.
Answer
- Glass is easy to clean
- Glass does not react with most chemicals
- Clear, hence possible to see reactions and changes
- Glass does not react with most chemicals
- Clear, hence possible to see reactions and changes
Question 6
Define the following terms:
I. A saturated solution.
II. Crystallization.
Answer
I. A saturated solution.
A solution that cannot take any more solute at any given temperature
II. Crystallization.
Formation of crystals from a saturated solution
A solution that cannot take any more solute at any given temperature
II. Crystallization.
Formation of crystals from a saturated solution
Question 7
Describe how copper (II) sulphate crystals can be obtained from copper (II) sulphate solution.
Answer
- Heat copper (II) sulphate solution to evaporate excess water /to obtain a saturated solution
- Cool the saturated solution to obtain copper (II) sulphate crystals.
- Dry the crystals between filter papers.
- Cool the saturated solution to obtain copper (II) sulphate crystals.
- Dry the crystals between filter papers.
Question 8
Study the table below and use it to answer the questions that follows. Letters are not the actual symbols of the elements
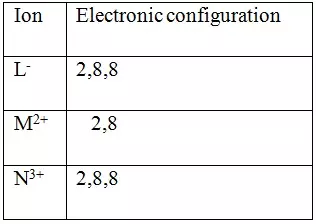
(a) Which elements belong to the same period of the periodic table?
(b) What is the formula of the compound formed by L and N?
(c) Compare the atomic and ionic radii of element L.
Answer
(a) L and M
(b) NL3
(c) The ion of L has a larger radius than the atom of L.
(b) NL3
(c) The ion of L has a larger radius than the atom of L.
Question 9
Write the chemical fomular of the following compounds.
(i) Sodium sulphate
(ii) Magnesium hydroxide
(iii) Calcium nitrate.
Answer
(i) Sodium sulphate
Na2SO4
(ii) Magnesium hydroxide
Mg(OH)2
(iii) Calcium nitrate
Ca(NO3)2
Na2SO4
(ii) Magnesium hydroxide
Mg(OH)2
(iii) Calcium nitrate
Ca(NO3)2
Question 10
State the reason why carbon ( iv) oxide is used by ice cream vendors instead of ordinary ice.
Answer
- Dry carbon (IV) oxide evaporates leaving no wetness.
- Carbon (IV) oxide is a better coolant
- Carbon (IV) oxide is a better coolant
Question 11
A student set-up the apparatus below in order to determine the percentage by volume of oxygen in air.
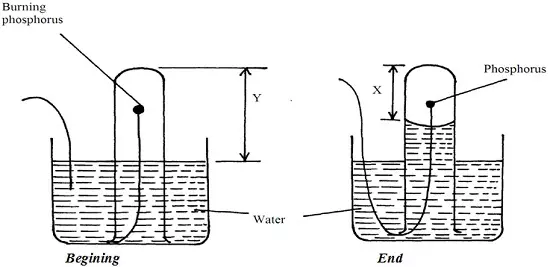
a) Why did water rise when the reaction had stopped?
b) The student wrote the expression for the percentage by volume of oxygen in air as
[(y-x)÷y] x 100
Why was the volume of oxygen calculated using the above expression incorrect?
c) What should have been done after the reaction had stopped in order to get a correct volume.
Answer
a) To occupy the space that was initially occupied by oxygen gas
b) Because oxides of phosphorous formed still occupy space enviously occupied by oxygen.
(P2O5, P2O3)
c) Let all the fumes dissolve in water before final reading is taken
b) Because oxides of phosphorous formed still occupy space enviously occupied by oxygen.
(P2O5, P2O3)
c) Let all the fumes dissolve in water before final reading is taken
Question 12
Explain how you would obtain solid lead carbonate from a mixture of lead carbonate and sodium chloride.
Answer
- Add water to the mixture and stir to dissolve sodium chloride
- Filter to obtain sodium chloride as a filtrate and lead carbonate as a residue
- Wash the residue and dry it between filter paper
- Filter to obtain sodium chloride as a filtrate and lead carbonate as a residue
- Wash the residue and dry it between filter paper
Question 13
Aluminium metal is a good conductor and is used for overhead cables. State any other two properties that make aluminium suitable for this use.
Answer
Aluminium does not rust
Aluminium has more delocalized electrons hence a better conductor of electricity.
Aluminium has more delocalized electrons hence a better conductor of electricity.
Question 14
In an experiment, a test tube of chlorine gas was inverted in water as shown in the diagram. It was then left to stand in sunlight for one day.
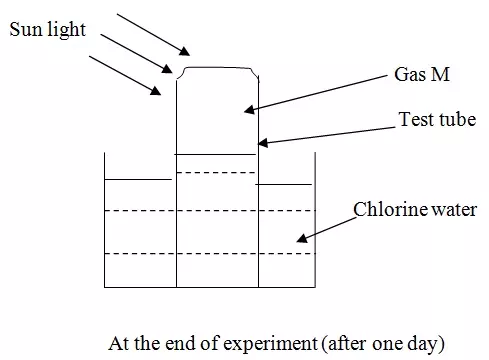
After one day, a gas M was found to have collected in the test tube as shown above.
(i) Identify gas M.
(ii) Suggest whether the PH of the solution in the beaker would increase or decrease after one day. Give an explanation.
(iii) The colour of chlorine water was observed to have changed from pale yellow to colourless after one day. Explain.
(iv) Write an equation to support your answer in (iii) above.
(v) State and explain the observation made when a moist blue litmus paper was placed at the mouth of the test tube containing chlorine gas.
(vi) Give two uses of chlorine gas.
Answer
(i) Oxygen gas
(ii)
- PH would decrease.
- Chloric (i) acid (unstable) decompose to hydrochloric acid, which is a strong acid.
(iii) Chloric (i) acid is yellow in colour. When exposed to sun light it decomposes to HCl acid and oxygen gas. HCl acid is colourless.
(iv) 2HOCl → 2HCl (aq) + O2 (g)
(v) The litmus paper turned red then white. It turned red because of the presence of hydrogen ions then white/ breached by chloric (i) acid though oxidation.
(vi)
- Used in the manufacture of hydrochloric acid
- Used in making breaches
- Used to make plastics (pvc)
- Used to kill microorganism in water treatment
(ii)
- PH would decrease.
- Chloric (i) acid (unstable) decompose to hydrochloric acid, which is a strong acid.
(iii) Chloric (i) acid is yellow in colour. When exposed to sun light it decomposes to HCl acid and oxygen gas. HCl acid is colourless.
(iv) 2HOCl → 2HCl (aq) + O2 (g)
(v) The litmus paper turned red then white. It turned red because of the presence of hydrogen ions then white/ breached by chloric (i) acid though oxidation.
(vi)
- Used in the manufacture of hydrochloric acid
- Used in making breaches
- Used to make plastics (pvc)
- Used to kill microorganism in water treatment
Question 15
A student set up the experiment below to collect gas K. the glass wool was heated before heating the magnesium coil.
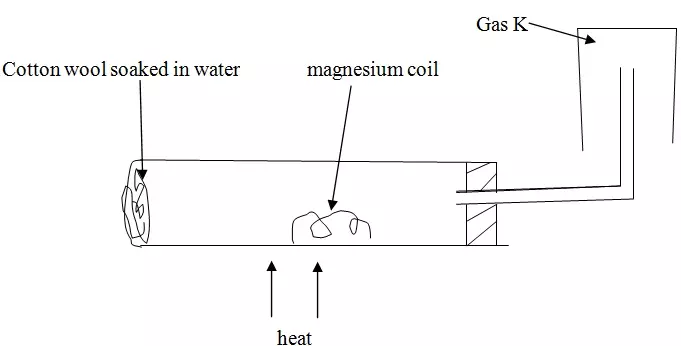
(a) Explain why it was necessary to heat the moist cotton wool before heating the magnesium.
(b) Identify gas K.
(c) What property of gas K makes it possible to be collected by the method shown?
(d) Write a chemical equation for the reaction that produced gas K.
Answer
(a) To produce steam this will react with magnesium. Heating magnesium first will make magnesium to react with oxygen.
(b) Hydrogen gas
(c) It's lighter than air
(d) Mg + H2O → MgO + H2
(b) Hydrogen gas
(c) It's lighter than air
(d) Mg + H2O → MgO + H2
Question 16
The diagram represents two methods of gas collection in the laboratory.
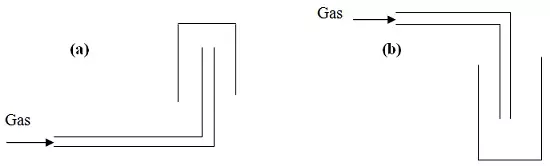
(i) Name the methods of gas collection above.
(ii) Which method is suitable for collecting dry carbon (IV) oxide gas? Give a reason.
Answer
(i). Name the methods of gas collection above.
(a) Upward delivery
(b) Downward delivery
(ii) Which method is suitable for collecting dry carbon (IV) oxide gas? Give a reason.
- Downward delivery
- Carbon (IV) oxide is denser than air.
(a) Upward delivery
(b) Downward delivery
(ii) Which method is suitable for collecting dry carbon (IV) oxide gas? Give a reason.
- Downward delivery
- Carbon (IV) oxide is denser than air.
Question 17
The curves bellow represent the variation of temperature with time when pure and impure samples of a solid were heated separately.
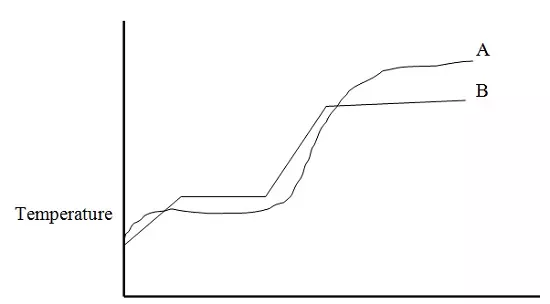
(a) Which curve represents the variation in temperature for pure solid? Explain.
(b) State the effect of an impurity on the melting and boiling points of a pure substance.
Answer
(a) Which curve represents the variation in temperature for pure solid? Explain.
B, has sharp melting and boiling point
(b) State the effect of an impurity on the melting and boiling points of a pure substance.
Impurity lowers the melting point and raises the boiling point
B, has sharp melting and boiling point
(b) State the effect of an impurity on the melting and boiling points of a pure substance.
Impurity lowers the melting point and raises the boiling point
Question 18
i) Cars in Mombasa are found to rust faster than cars in Nairobi. Explain.
ii) State one disadvantage of rusting.
Answer
i) Mombasa is salty. Salt accelerates rusting.
ii) Causes wear and tear
ii) Causes wear and tear
Question 19
The PH of a soil sample in a given area was found to be 5.5. An Agricultural officer the addition of lime (calcium oxide). State the function of lime in the soil.
Answer
- Neutralizes the soil.
- Adds calcium to the soil.
- Adds calcium to the soil.
