θ0 = (273 + θ) K, and T (K) = (T – 273) °C.
Example 1
A gas in a fixed volume container has a pressure of 1.6 × 105 Pa at a temperature of 27°C. What will be the pressure of the gas if the container is heated to a temperature of 277 °C?
Solution
Since law applies for Kelvin scale, convert the temperature to kelvin
T1 = 27 °C = (273 + 27) K = 300 K
T2 = 227 °C = (273 + 277) = 550 K
P1 / T1 = P2 / T2, therefore P2 = (1.6 × 105) × 550 / 300 = 2.93 × 105 Pa.
At 20 °C, the pressure of a gas is 50 cm of mercury. At what temperature would the pressure of the gas fall to 10 cm of mercury?
Solution
P / T = constant, P1 / T1 = P2 / T2, therefore T2 = (293 × 10) / 50 = 58.6 K or (– 214.4 °C.
Charles law
Charles law states that "the volume of a fixed mass of a gas is directly proportional to its absolute temperature (Kelvin) provided the pressure is kept constant". Mathematically expressed as follows,V1 / T1 = V2 / T2
Example 3
A gas has a volume of 20 cm3 at 27 °C and normal atmospheric pressure. Calculate the new volume of the gas if it is heated to 54 °C at the same pressure.
Solution
Using, V1 / T1 = V2 / T2, then V2 = (20 × 327) / 300 = 21.8 cm3.
Example 4
0.02m3 of a gas is at 27 °C is heated at a constant pressure until the volume is 0.03 m3. Calculate the final temperature of the gas in °C.
Solution
Since V1 / T1 = V2 / T2, T2 = (300 × 0.03) / 0.02 = 450 K or 177 °C
Boyle's law
Boyle's law states that the pressure of a fixed mass of a gas is inversely proportional to its volume provided the temperature of the gas is kept constant. Mathematically expressed as,P1 V1 = P2 V2
Example 5
A gas in a cylinder occupies a volume of 465 ml when at a pressure equivalent to 725 mm of mercury. If the temperature is held constant, what will be the volume of the gas when the pressure on it is raised to 825 mm of mercury?
Solution
Using, P1 V1 = P2 V2, then V2 = (725 × 465) / 825 = 409 ml.
Example 6
The volume of air 26 cm long is trapped by a mercury thread 5 cm long as shown below. When the tube is inverted, the air column becomes 30 cm long. What is the value of atmospheric pressure?
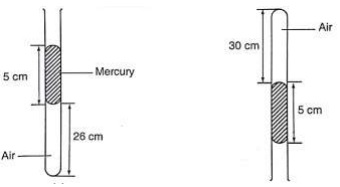
Before inversion, gas pressure = atm. Pressure + h p g
After inversion, gas pressure = atm. Pressure - h p g
From Boyle's law, P1 V1 = P2 V2, then let the atm. Pressure be ‘x',
So (x + 5) 0.26 = (x – 5) 0.30
0.26x + 1.30 = 0.3x - 1.5, x = 2.8/ 0.04 = 70 cm.
A general gas law
Any two of the three gas laws can be used derive a general gas law as follows,P1 V1 / T1 = P2 V2 / T2 or
P V / T = constant – equation of state for an ideal gas.
Example 7
A fixed mass of gas occupies 1.0 × 10-3 m3 at a pressure of 75 cmHg. What volume does the gas occupy at 17.0 °C if its pressure is 72 cm of mercury?
Solution
P V / T = constant so V1 = (76 × 1.0 × 10-3 × 290) / 273 × 72 = 1.12 × 103 m3.
Example 8
A mass of 1,200 cm3 of oxygen at 27 °C and a pressure 1.2 atmosphere is compressed until its volume is 600 cm3 and its pressure is 3.0 atmosphere. What is the Celsius temperature of the gas after compression?
Solution
Since P1 V1 / T1 = P2 V2 / T2, then T2 = (3 × 600 × 300) / 1.2 × 1,200 = 375 K or 102 °C.

