Question 1
The water level in a burette is 40.6cm3. 50 drops of water each of volume 0.2cm3 are added to the water in the burette. What is the final reading of the burette?
Answer
Additional volume = 50x0.2= 10.0cm3
Final reading = 40.6 - 10.0
= 30.6cm3
Final reading = 40.6 - 10.0
= 30.6cm3
Question 2
Figure 1 shows a U-tube manometer used to measure lung pressure.
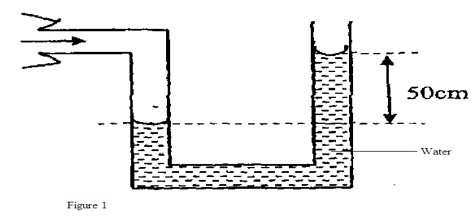
Determine the lung pressure given that atmospheric pressure 1.03 x 1 Nm-2 and density of Water 1000 kgm3
Answer
Lung pressure = Atm. Pressure + pressure due to water column
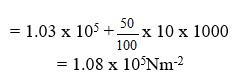
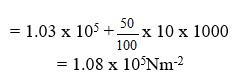
Question 3
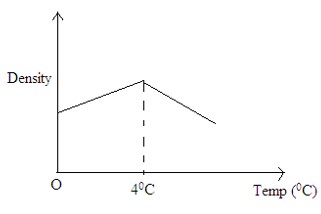
on the axes provided sketch density-temperature graph, when water is heated from a Temperature of 0°C to 10°C.
Answer
Question 4
The springs in figure 2 are identical
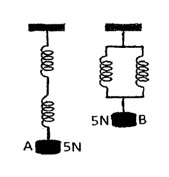
The extension produced in A is 4cm. What is the extension in B?
Answer
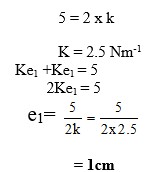
Question 5
State and explain how the motion of the smoke particles changes when the temperature Inside the smoke cell is lowered.
Answer
They slowdown/ speed reduced due to decrease in kinetic energy of the particles.
