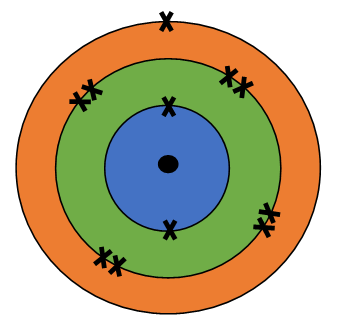
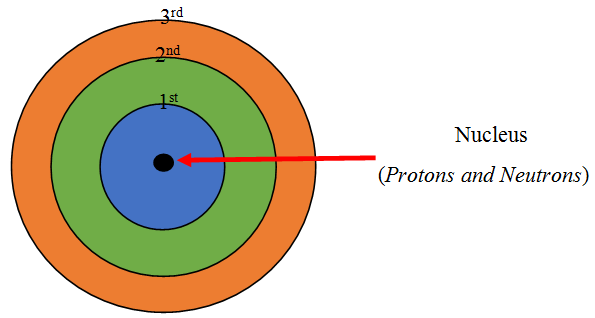
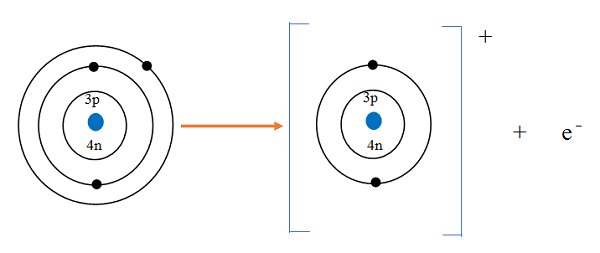
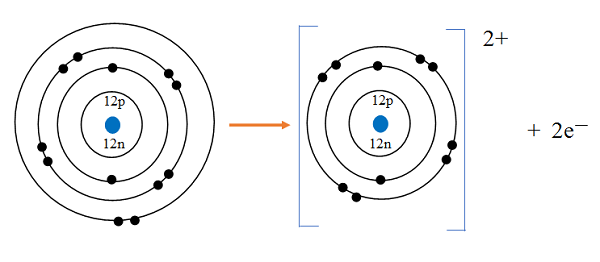
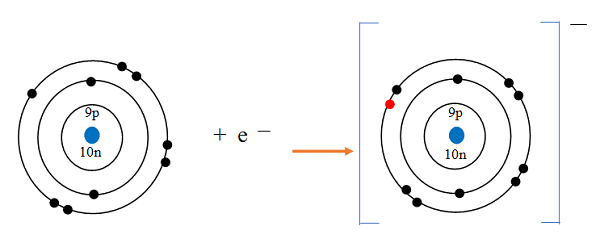
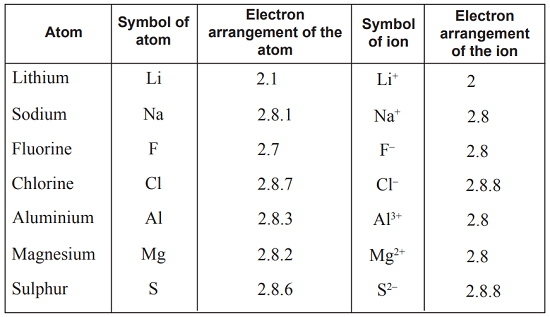
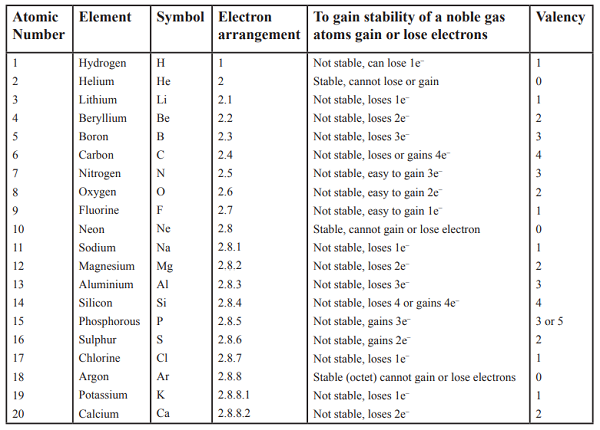
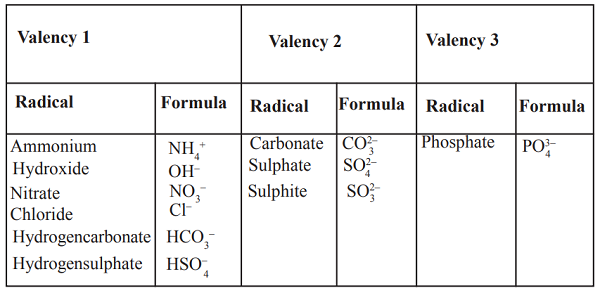
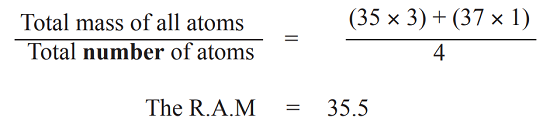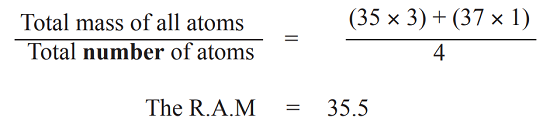The atomic number tells us
how many protons are there in a nucleus. It is denoted by letter Z.
It also tells us the number of electrons in an atom
Atomic Number = Number of Protons = Number of Electrons
The mass number is the sum of protons and neutrons, therefore it is always bigger than the atomic number. It is denoted by letter A.
Roughly the mass number is double the atomic number.
To get the number of neutrons, we just subtract the atomic number from the mass number, that is A – Z.
| Atom |
Symbol |
Number of Protons:
Atomic number, Z |
Neutrons |
Mass Number, A |
| Hydrogen |
H |
1 |
0 |
1 |
| Carbon |
C |
6 |
6 |
12 |
| Nitrogen |
N |
7 |
7 |
14 |
| Sodium |
Na |
11 |
12 |
23 |
| Chlorine |
Cl |
17 |
18 |
35 |
Example
Calculate the number of neutrons in a chlorine atom given that the atomic number, Z = 17 and mass number, A = 35
The number of neutrons
= A – Z
= 35 – 17
= 18
Usually, the atomic number, Z, and mass number A, of an atom of an element X can be written alongside the symbol of that element, one as a superscript and the other a subscript as shown below
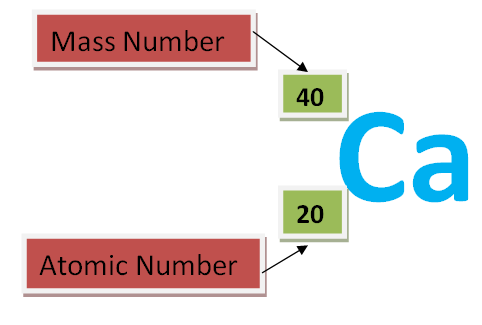
NOTE: The top number is referred to as the superscript and bottom number as the subscript.
Isotopes
Isotopes are atoms of the same element, which have the
same number of protons in the nucleus (atomic number) but
different mass numbers.
There are two examples below.
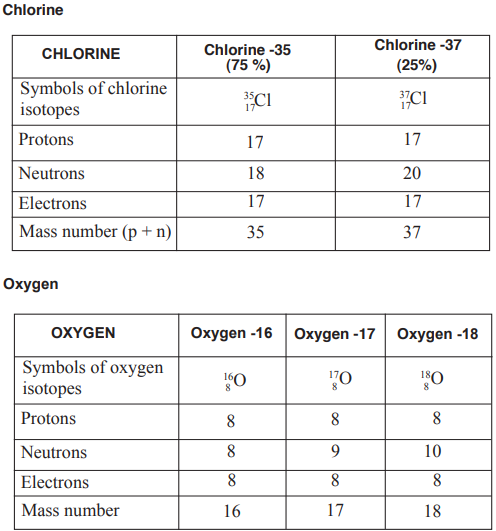
Relative Atomic Mass
Originally, the mass of a hydrogen atom was taken as the standard reference atom. Its atomic mass was arbitrarily fixed as one unit i.e H = 1.
The mass of any other element was found by comparing its mass with that of hydrogen. The idea was to find out how many times the atom of another element is as heavy as one atom of hydrogen hence
relative atomic mass (R.A.M)
Relative Atomic Mass = mass of 1 atom of the element divided by mass of 1 atom of hydrogen
For instance an oxygen atom (O), has a mass of 16. This means one oxygen atom is 16 times heavier than one atom of hydrogen.
NOTE
Relative atomic mass has no units. It is a ratio of two masses. The relative atomic masses are not whole numbers like mass numbers. This is because the abundance of isotopes of an element is different.
Example
Chlorine consists of two isotopes, chlorine – 35 and chlorine – 37 in the ratio 3 : 1. Calculate the relative atomic mass (R.A.M) of chlorine.
Suppose the sample contains 4 atoms of chlorine, in the ratio 3 : 1
3 atoms will each have a mass of 35
1 atom will have a mass of 37
The total mass of 35Cl = 35 × 3
While the total mass of 37Cl = 37 × 1
Therefore, the average mass of chlorine atoms will be