(ii) Suspension / Precipitates (solid-liquid mixture which do not dissolve)
Some solid substances do not dissolve in a liquid. They are said to be insoluble in the solvent. When an insoluble solid is put in liquid
- Some particles remain suspended/floating in the liquid to form a suspension /precipitate
- Some particles sink/settle to the bottom to form sediments after being allowed to stand
For instance, putting maize flour in water creates a white precipitate
(iii) Miscible (liquid-liquid mixtures)
Miscible liquids form a uniform mixture and do not form layers
The particles of one liquid are smaller than the particles of the other and therefore, the smaller particles occupy the spaces between the bigger particles
Examples: Water and Ethanol and miscible liquids, and kerosene and turpentine are also miscible
(iv) Immiscible (liquid-liquid mixtures)
Immiscible mixtures do not form uniform mixtures, they form layers.
The particles of one liquid cannot occupy the spaces between the particles of the other.
The heavier particles settle at the bottom. The less dense particles settle on top
For instance, Kerosene and water are immiscible
(iv) Solid-solid mixtures
An alloy is a uniform mixture of two metals formed on solidifying
The following table shows the common alloys of metals
| Alloy Name |
Constituents of the Alloy |
Uses of the Alloy |
| Brass |
Copper and Zinc |
Making screws and bulb caps |
| Bronze |
Copper and Tin |
Making clock springs, electrical contacts and copper coins |
| Soldier |
Lead and Tin |
Soldering, joining electrical contacts because of its low melting points and high thermal conductivity |
| Duralumin |
Aluminum, Copper and Magnesium |
Making aircraft, utensils, and windows frames because its light weight and corrosion resistant |
| Steel |
Iron, Carbon Manganese and other metals |
Railway lines, car bodies girders and utensils. |
| Nichrome |
nickel and Chromium |
Provide resistance in electric heaters, toasters, and ovens |
| German silver |
Copper, Zinc and Nickel |
Making coins |
Methods of Separating Mixtures
(i) Decantation
Through this method, sediments can be separated from a liquid by pouring out the liquid.
Experimentally, one can put some sand in a beaker. Add about 200cm3 of water. Allow sand to settle. Pour off water carefully into another beaker.
One will observe that sand settles at the bottom as sediments and less clean water is poured out.
(ii) Filtration
Filtration is the method of separating insoluble mixtures/particles/solids from a liquid.
In an experiment, one should fold a filter paper to fit well into a filter funnel. Place the funnel in an empty 250 cm3 beaker. Put one spatula end full of soil into 50cm3 of water. Stir. Put the soil/water mixture into the filter funnel.
From that experiment, one shall observe that clean water is collected below the filter funnel and soil remains above the filter paper.
PS:
(iii)Filtration is like improved decantation
(iii) Evaporation
This is a method of separating a solute/solid from its solution. This involves heating a solution (solvent and solute)to vaporize the solvent out of the solution mixture leaving pure solute/solid
For instance, a solution of salt and water is separated through evaporation. Soil and salt can also be separated through evaporation, first by adding them to water, filtering the soil, and the evaporating the water to be left with salt.
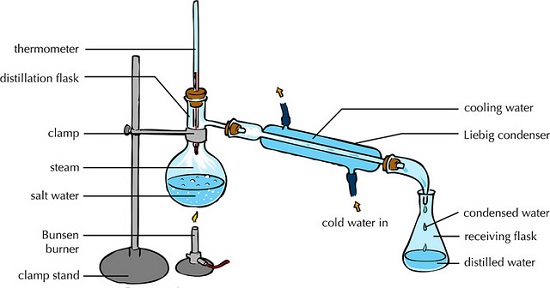
(iv) Distillation
This is an improved evaporation where both the solute and the solvent in the solution are separated /collected.
The process separates a solution into constituent solid solute and the solvent.
It involves heating the solution to evaporate/vaporize the solvent out. The solvent vapour is then condensed back to a liquid.
An example of this in an experiment would be to obtain copper (II) sulphate crystals and water from copper (II) sulphate solution.
The following illustration shows how the pro0cess is undertaken.
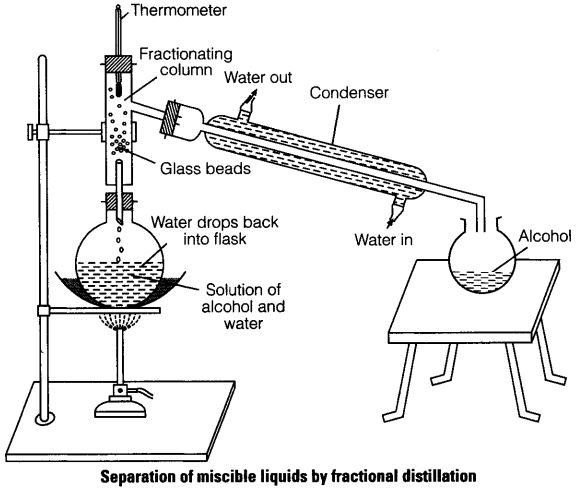
(v) Fractional Distillation
This method is an improved simple distillation used specifically to separate miscible mixtures with very close /near boiling points.
It is done in the following steps
a) Heating the mixture in a conical/round bottomed /flat bottomed flask
b) The conical/round bottomed /flat bottomed flask is connected to a long glass tube called
fractionating column.
NB: The
fractionating column offers areas of condensation for the less volatile pure mixture packed with glass beads/broken glass/ porcelain/ shelves to increase the surface area of condensation
c) When the vapours rise they condense on the glass beads/broken glass /porcelain / shelves which become hot. When the temperature of the glass beads/broken glass/porcelain/shelves is beyond the boiling point of the less volatile pure substance, the pure substance rise and condensation take place on the glass beads/broken glass/porcelain/shelves at a higher level on the fractionating column. The less volatile pure substance trickles/drips back down the fractionating column or back into the conical/round bottomed /flat bottomed flask to be heated again
d) The fractionating column is connected to a
Liebig condenser. The Liebig condenser has a cold water inlet and outlet circulation. The more volatile mixture that reach the top of the fractionating column is condenses by the Liebig condenser into a receiver. It is collected as the first fraction.
e) At the top of the fractionating column, a thermometer is placed to note/monitor the temperature of the boiling mixtures.
Pure substances have constant/fixed boiling point. When one mixture is completely separated, the thermometer reading rises.
f) When no more of the more volatile solvent is being separated, the mercury/alcohol level in the thermometer rises. The second /subsequent fractions are collected in the receiver after noting a rise the mercury/alcohol level in the thermometer.
g) Each fraction collected should be confirmed from known physical/chemical properties/characteristic.
The following is a set-up showing how the experiment is undertaken when separating water from ethanol.
Fractional distillation is used industrially. It is used in distillation of crude oil in an oil refinery, as well as distillation of air
(vi) Separation of immiscible Liquids (Using a separating funnel)
This method is used to separate liquids that form layers on mixing.
Immiscible mixture arrange themselves according to their densities.
The denser liquid sink to the bottom. The less dense liquid floats on the denser one. Immiscible mixtures can be separated from each other by using a separating funnel.
Paraffin and water can be separated using this method.
The apparatus are set up as follows, with the denser liquid being collected first, and the less dense one collected after
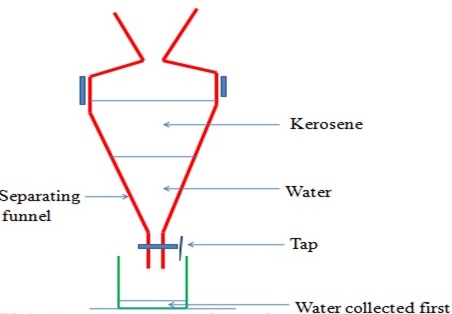
(vii) Sublimation/deposition
Some solids on heating do not melt to a liquid but change directly to a gas. The process by which a solid changes to a gas is called
sublimation. The gas cools back and changes directly to a solid. The process by which a gas changes to a solid is called deposition. Sublimation and deposition therefore are the same but opposite processes.
Among substances that undergo sublimation include;
iodine, carbon (IV) oxide, benzoic acid, ammonium, camphor, and iron (III) chloride.
Mixtures between those substances can be separated through sublimation and deposition
Different experiments can be used to show sublimation and deposition in separation of substances.
(viii) Chromatography
Chromatography is a method of separating components of a solution mixture by passing it through a medium where the different components move at different rates. The medium through which the solution mixture is passed is called absorbent material.
Paper chromatography is a method of separating colored dyes by using paper as the
absorbent material.
Since dyes are insoluble/do not dissolve in water, ethanol and propanone are used as suitable solvents for dissolving the dye
Practically, a simple paper chromatography involve placing a dye/material on the absorbent material, adding slowly a suitable soluble solvent on the dye/material using a dropper, the solvent spread out on the absorbent material carrying the soluble dye away from the origin.
The spot on which the dye is initially/originally placed is called
baseline. The farthest point the solvent spread is called
solvent front.
The farthest a dye can be spread by the solvent depend on:
(i) Density of the dye - the denser the dye, the less it spread from the basely by the solvent.
(ii) Stickiness of the dye-some dyes sticks on the absorbent material more than other thus do not spread far from baseline.
When a drop of ink is placed on an absorbent material it sticks. On adding an eluting solvent, it dissolves the dye spread out with it. The denser and sticky pure dye move least. The least dense/sticky pure dye move farthest. A pure dye will produce the same chromatogram/spot if the same eluting solvent is used on the same absorbent material.
The chromatogram of pure dyes A, B ,C and a dye mixture D is shown below
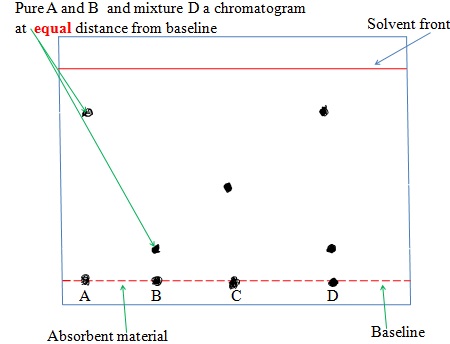
(ix) Solvent Extraction
Solvent extraction is a method of separating oil from nuts/seeds. Most nuts contain oil. First the nuts are crushed to reduce their size and increase the surface area. A suitable volatile solvent is added. The mixture is filtered. The filtrate solvent is then allowed to crystallize leaving the oil/fat. If a filter paper is rubbed/smeared with the oil/fat, it becomes translucent. This is the test for the presence of oil/fat.
(x) Crystallization
This is the process of using solubility of a solute/solid to obtain the solute/solid crystals from a saturated solution by cooling or heating the solution
A crystal is the smallest regular shaped particle of a solute. Every solute has unique shape of its crystals.
Some solutions form crystals when heated. This is because less solute dissolves at higher temperature. Some other solutions form crystals when cooled. This is because less solute dissolves at lower temperature.
For instance, one can crystallize copper (II) sulphate solution into its hydrated crystals
Experiment for crystallization could be performed as follows;
Place about one spatula full of hydrated copper sulphate (VI) crystals into 200cm3 of distilled water in a beaker. Stir. Continue adding a little more of the hydrated copper sulphate (VI) crystals and stirring until no more dissolve. Decant/filter. Cover the filtrate with a filter paper. Pierce and make small holes on the filter paper cover. Preserve the experiment for about seven days.