1. Measuring cylinder
Measuring cylinders are apparatus used to measure volume of liquid/ solutions. They are calibrated/ graduated to measure any volume required to the maximum. Measuring cylinders are named according to the maximum calibrated/graduated volume.
An example is the 50ml-measuring cylinder can hold maximum calibrated/graduated volume of 50 milliliters or 50 cubic centimetres

2. Burette
A burette is a long and narrow/thin apparatus used to measure small accurate and exact volumes of a liquid solution. It must be clamped first on a stand before being used. It has a tap to run out the required amount out.

3. Pipette and pipette filler
Pipette is a long and narrow/thin apparatus that widens at the middle used to measure and transfer small very accurate/exact volumes of a liquid solution
Pipette filler is used to suck in a liquid solution into a pipette instead of using the mouth. It has a suck, adjust and eject button for ensuring the exact volume is attained

4. Volumetric flask
A volumetric flask is thin /narrow but widens at the base/bottom. It is used to measure very accurate/exact volumes of a liquid solution
The maximum calibration / graduation mark is a visible ring, and are named according to their calibrated volume
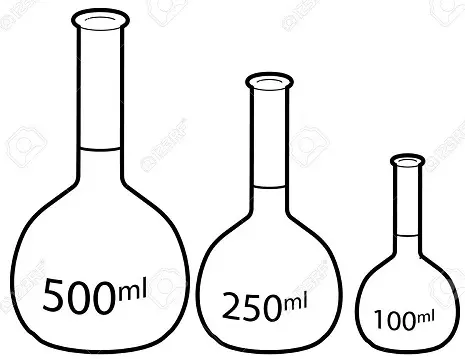
In order to adjust the volume to the calibration, a dropper or a teat pipette can be used.