- It occurs as water and in petroleum.
Lab Preparation of Hydrogen
An experiment is conducted as follows;- Put Zinc granules in a round/flat/conical flask
- Add dilute sulphuric acid or Hydrochloric acid
- Add about 3cm3 of copper (II) sulphate solution.
- Collect the gas produced over water as in the set up below
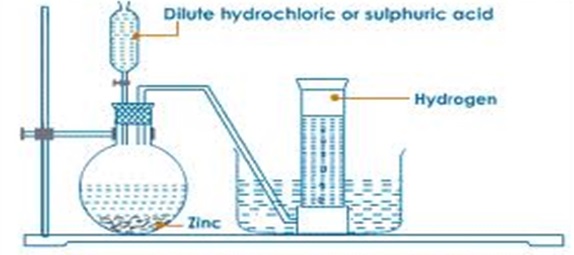
- Discard the first gas jar and collect several gas jars
- Zinc reacts with dilute sulphuric (VI)/hydrochloric acid to form a salt and produce hydrogen gas.
- When the acid comes into contact with the metal, there is rapid effervescence/ bubbles /fizzing are produced and a colourless gas is produced that is collected
- The gas is collected over water because it is insoluble in water
- The gas is collected through downward displacement of air/upward delivery because it is less dense than air.
- The first gas jar is impure because it contains air that was present in the apparatus.
- Copper (II) sulphate is used as a catalyst
Zinc + Hydrochloric acid
 Zinc chloride + Hydrogen
Zinc chloride + HydrogenZn(s) + 2HCl (aq)
 ZnCl2 (aq) + H2 (g)
ZnCl2 (aq) + H2 (g)NB: Similar results can be obtained when iron and magnesium are used in place of zinc.
NB: Hydrogen cannot be prepared from reaction of Nitric (V) acid and a metal because this acid is a strong oxidizing agent and it oxidizes hydrogen gas to water
NB: Hydrogen cannot be prepared from reaction of Dilute sulphuric (VI) acid with calcium/Barium/Lead because sulphate salts formed are insoluble. Once formed, they cover/coat the unreacted calcium/Barium/Lead stopping further reaction and producing very small amount/volume of hydrogen gas.
NB: Hydrogen cannot be prepared from reaction of Dilute acid with sodium/potassium because the reaction is explosive
Physical Properties of Hydrogen gas
- Hydrogen is a neutral, colourless and odorless gas
- It is insoluble in water thus can be collected over water.
- It is the lightest known gas. It can be transferred by inverting one gas jar over another.
Chemical Properties of Hydrogen gas
a. Burning
A. Hydrogen does not support burning/combustion. When a burning splint is inserted into a gas jar containing Hydrogen, the flame is extinguished /put off.B. Pure dry hydrogen burn with a blue quiet flame to form water. When a stream of pure dry hydrogen is ignited, it catches fire and continues to burn with a blue flame.
C. Impure (air mixed with) hydrogen burns with an explosion. Small amount/ volume of air mixed with hydrogen in a test tube produce a small explosion as a “pop” sound. This is the confirmatory test for the presence of Hydrogen gas. A gas that burns with a “pop” sound is confirmed to be Hydrogen.
b. Redox in terms of Hydrogen transfer
Redox can also be defined in terms of Hydrogen transfer where;- Oxidation is removal of Hydrogen
- Reduction is addition of Hydrogen
- Redox is simultaneous addition and removal of Hydrogen
Example;
- When a stream of dry hydrogen gas is passed through black copper (II) oxide, hydrogen gas gains the oxygen from copper (II) oxide.
- Black copper (II) oxide is reduced to brown copper metal.
Copper (II) Oxide + Hydrogen
 Copper + Hydrogen gas
Copper + Hydrogen gasCuO (s) + H2(g)
 Cu(s) + H2O(l)
Cu(s) + H2O(l)- Black copper (II) oxide thus the Oxidizing agent.
- Hydrogen gas is oxidized to Water.
Oxygen + Hydrogen
 Water
WaterO2(g) + 2H2(g)
 2H2O (l)
2H2O (l)- Hydrogen is the Reducing agent.
c. Water as an Oxide of Hydrogen
Burning is a reaction of an element with OxygenThe substance formed when an element burn in air is the oxide of the element.
When hydrogen burns, it reacts/ combines with Oxygen to form the oxide of Hydrogen
The oxide of Hydrogen is called water
Hydrogen is first dried because a mixture of Hydrogen and air explode
The gas is then ignited
The products condense on a cold surface/flask containing a freezing mixture.
A freezing mixture is a mixture of water and ice.
The setup below illustrates burning of hydrogen to show that water is an oxide of hydrogen
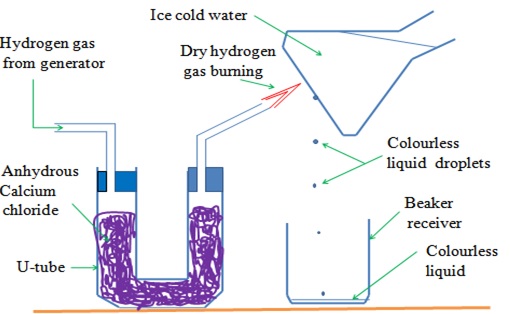
Uses of Hydrogen gas
1. Hydrogenation or Hardening of unsaturated vegetable oils to saturated fats/margarine in presence of Nickel catalyst and about 150oC2. In weather forecast balloons
3. In the Haber process for the manufacture of Ammonia
4. In the manufacture of Hydrochloric acid.
5. As rocket fuel.
6. In oxy-hydrogen flame for welding.

