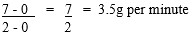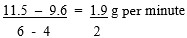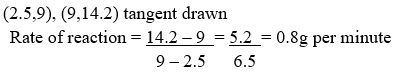Question 1
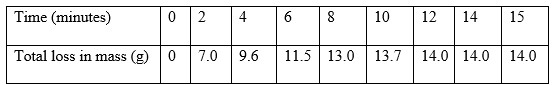
Excess zinc granules was added to a beaker containing 100cm3 of dilute sulphuric(vi) acid. The beaker was then placed on a balance and the total loss in mass recorded after every two minutes as shown in the table below.
- Why was there loss in mass?
- Calculate the average rate of loss in mass between
- 0 and 2 minutes
- 4 and 6 minutes
- Explain the difference in the average rates in b(i)and b(ii) above
- Write a balanced equation for the reaction rate above could be increase.
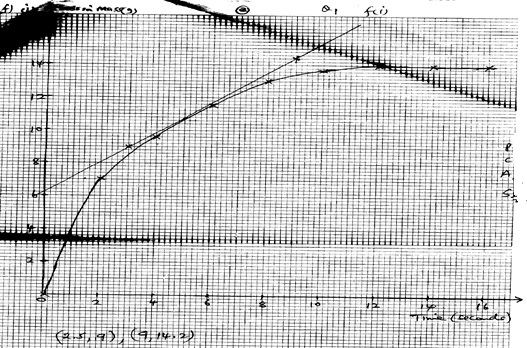
- Plot the graph of total loss in mass(g) versus time (minutes).
- Use the graph to calculate the rate of the reactions when t = 5 minutes.
Answer
- Because a gas evolved and escaped
-
- The reaction was faster between the 0 and 2 minutes than 4 and 6 minutes because the concentration of reactants ( H2SO4) was higher between 0 and 2 minutes than 4 and 6 mins.
-

Question 2
- State how burning can be used to distinguish between prepane and propyne. Explain your answer.
- Draw the structural formular of the 4th member of the homologous series in which propyne is a member.
Answer
- When ignited propane burns with a blue flame because it is a saturated hydrocarbon (alkane) while propyne burns with a yellow smoky flame because it is an unsaturated hydrocabon (alkyne).
-
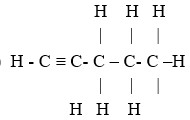
Question 3
The flow chart below shows a series of reactions starting with propan-l-ol. Study it and answer the questions that follow.
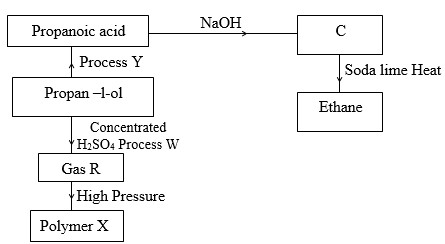
- Name:
- Process Y
- Substances R and C
R
C - Process W
- Polymer X
- Write a balanced chemical equation for the combustion of propane.
- State one use of polymer X
Answer
-
- Process Y - oxidation
-
R - Propene (Prop– l-ene)
C - Sodium propnoate - Process W - Dehydration
- Polymer X - polypropene
-

- Used to manufacture plastic ropes
Question 4
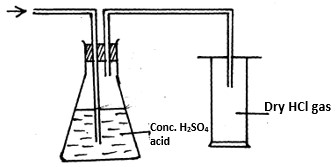
The set up below was used to prepare dry hydrogen chloride gas.
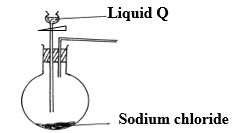
- Complete the diagram to show how dry hydrogen chloride gas is collected.
- Identify liquid Q
- Write a balanced equation for the reaction that produces hydrogen chloride gas in the above experiment.
- State the effect of dry hydrogen chloride gas on
- Dry litmus paper
- Wet litmus paper
Answer
-
- liquid Q - Concentrated Sulphuric (vi) acid
-

- State the effect of dry hydrogen chloride gas on
- Dry HCl gas has no effect on red and blue litmus.
- Dry HCl gas turns blue litmus paper red but has no effect on red litmus.
Question 5
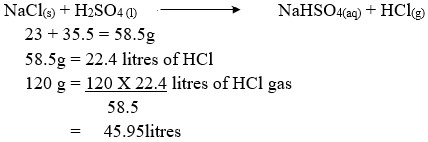
Calculate the volume of hydrogen chloride gas produced if 120g of sodium chloride was used with excess of liquid Q at S.T.P .( Na= 23,Cl=35.5,H=1.0, S = 3) molar gas volume = 22.4 litres at stp.
Answer