Question 1
The grid below shows part of the periodic table. Use it to answer the questions that follow.
The letters do not represent actual symbols.
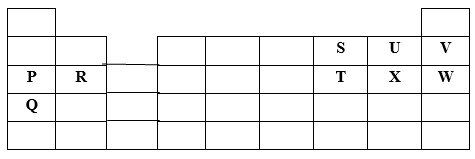
(a) Which of the elements has the highest atomic radius? Explain
(b) Identify the most reactive Oxidizing agent. Explain.
(c) Compare the atomic radius of P and R. Explain
(d) Give the formula of one stable ion with an electron arrangement of 2.8 which is:
- Negatively charged divalent ion
- A Positively charged monovalent
(f) Write the formula of the compounds formed between.
- Element R and X
- Give one property of the structure formed when R and X bond.
Answer
(a) Q - This is because it has the highest number of energy levels.
(b) U - This is because U has the highest nuclear change due to its small atomic radius among the non metals.
(c) P has bigger atomic radius than R. This is because P has higher nuclear charge than R
(d)
P = 18
N = 22
(f)
(b) U - This is because U has the highest nuclear change due to its small atomic radius among the non metals.
(c) P has bigger atomic radius than R. This is because P has higher nuclear charge than R
(d)
- S2-
- P+
P = 18
N = 22
(f)
- RX2
- (a) Compounds with the above structures are soluble in water but insoluble in organic conduct.
(b) Compounds with the above structure conduct electricity in molten and aqueous state but they are non conductors in solid state.
(c) Compounds with the above structure exist in crystalline form.
Question 2
Sodium hydroxide pellets were accidentally mixed with sodium chloride-18.2g of the mixture was dissolve in water to make one liter of solution. 100cm3 of the solution was neutralized by 50cm3 of 0.45M Sulphuric acid.
- Write an equation for the reaction that took place
- Calculate the;
I. Number of moles of the substance that reacted with sulphuric acid
II. Number of moles of the substance that would react with sulphuric acid in the one litre of solution
III. Mass of the unreacted substance in the one litre of solution.
(H = 1.0, Na = 23.0, Cl = 35.5, 0 = 16.0)
Answer
- 2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 22H2O(1)
- I.
II.
III.
Question 3
The diagram below shows an incomplete set-up used to prepare and collect ammonia gas.
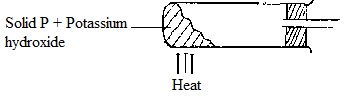
i) Name solid P.
ii) Complete the diagram to show how a dry sample of ammonia gas can be collected.
Answer
i) NH4CL(s) / any ammonium salt
ii)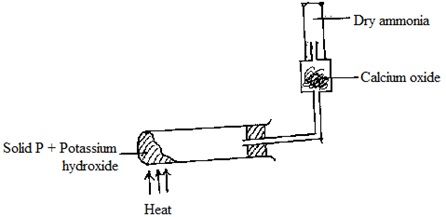
ii)

Question 4
The scheme below was used to prepare a cleansing agent. Study it and answer the questions that follow
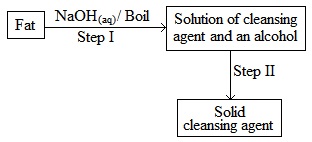
- What name is given to the type of cleansing agent prepared by the method above?
- Name one chemical substance added in step II.
- What is the purpose of adding the chemical substance named in a (ii) above?
- Name any other suitable substance that can be used in step I.
Answer
- Soap
- Alcohol
- Cleansing agent
- Sodium Hydroxide (NaOH)
