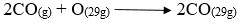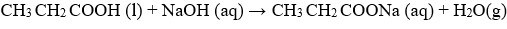Question 1
The flow diagram below shows some reactions starting with propanol. Study it and use it to answer the questions that follow.
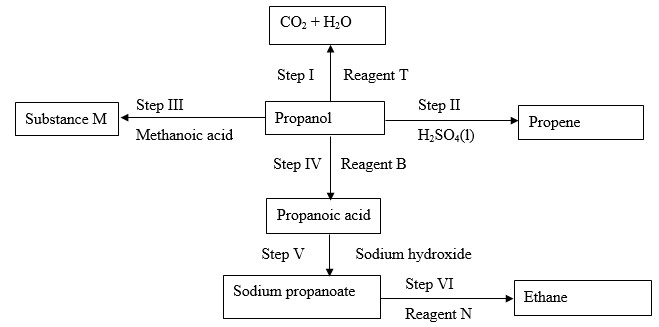
- Name the process in step
- I
- III
- IV
- Write an equation for the reaction in step
- I
- V
- Give the name and structural formula of substance M
- Name
- Structural formula
- Name the reagent
- B
- N
- State the condition necessary for reaction in step II
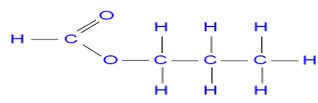
- The diagram below shows the structure of a detergent

- Identify the detergent
- A sample of water was found to contain magnesium ions. Explain why the detergent above is a suitable reagent to be used in the water.
Answer
-
- I - Combustion
- II - Esterification
- IV - oxidation
-
-
-
- Name - propylmethanoate
-
-
- B - Acidified potassium manganate(VII) or Acidified potassium dichromate (VI) accept the respective formulaes.
- N - Soda lime
- 170 – 1800C/ reject high temperature of temparatures out of range.
-
- Soapless
- Magnesium ions make water hard. The detergent forms lather easily with hard water and doesn’t form scum.
Question 2
The table below shows properties of four substances. Study it and use it to answer the questions that follow.
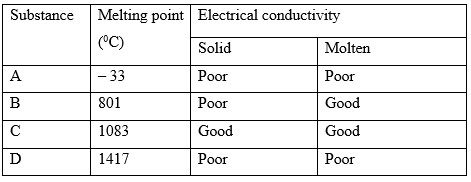
- Identify the structure in the following
- A
- C
- Explain the conductivity in substance B
- Give a reason why substance D has a high melting point.
Answer
-
- A - Molecular/ simple molecular
- C - Giant metallic/ metallic
- In solid state the ions are in fixed state hence a non-conductor. In molten state the ions dissociate and become mobile hence it conducts electricity.
- It has covalent bonds/ giant covalent structure which are strong throughout the structure hence a lot of energy is required to break them.
Question 3
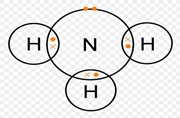
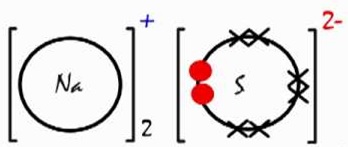
Draw dot and cross diagram showing bonding in the following;
- Ammonia gas (N=7, H=1)
- Sodium sulphide ( Na=11, S=16)
Answer
Question 4
Explain the following
- Magnesium and sulphur are in the same period of the periodic table. However, magnesium oxide is a solid while sulphur (IV) oxide is a gas at room temperature (Mg = 12, S=16, O=8).
- Ethanol is molecular but it dissolves in water
- Solid copper metal is a better electrical conductor than molten copper.
Answer
- Magnesium oxide has strong ionic bonds hence ions are held strongly. Sulphur (IV) oxide has molecules held together by weak van der waals forces.
- Ethanol is molecular but it dissolves in water
Ethanol has hydrogen bonds which make it polar and soluble in water - In molten state the electrons have increased kinetic energy which make them move randomly reducing their conductivity.
Question 5
The diagram below shows the set-up used to test a property of carbon in the laboratory. Study it and use it to answer the questions that follow.
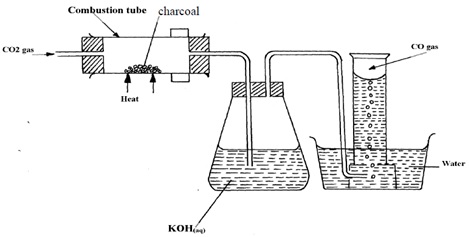
- State the role of potassium hydroxide solution.
- Write an equation for the reaction in the combustion tube.
- State the property of carbon being investigated.
Answer
- To absorb unreacted carbon (IV) oxide
- CO2(g) + C(s) → 2CO(g)
- Reducing property