Question 1
The elements X and Y have electronic configuration 2:8.3 and 2.6 respectively.
- To which group and period does Y belong?
- If the two reacts, what is the formula of the compound formed
Answer
- Group VI, period 2
- A2B3
Question 2
The empirical formular of a compound is CH2 and it has a molecular mass of 42.
- What is the molecular formular of this compound?
- To which group of hydrocarbons does the compound formed in (a) above belong?
- Draw the structural formula of the third member of this series and give its IUPAC name
Answer
-
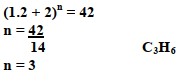
- alkenes
-
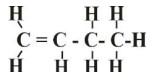
But–1–ene Or But-2-ene
Question 3
Excess carbon (II) oxide gas was passed over heated sample of an oxide of iron. Study it and answer the questions below using data provided below
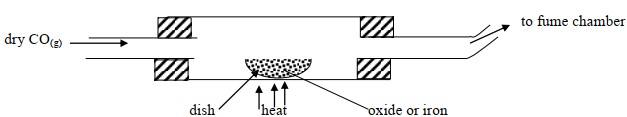
Mass of empty dish = 10.98 g
Mass of empty dish + oxide of iron = 13.14g
Mass of empty dish + Residue = 12.66g
Determine the simplest formula of the oxide of iron
(Fe=56, 0=16)
Answer
Mass of Iron 12.66- 10.98 = 1.68
Mass of oxygen 13.14-10.98 = 2.16g
Mass of oxygen be 2.16 – 1.68 = 0.48g
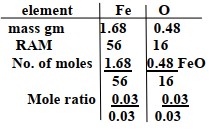
Mass of oxygen 13.14-10.98 = 2.16g
Mass of oxygen be 2.16 – 1.68 = 0.48g

Question 4
Study the table below and answer the questions that follows.
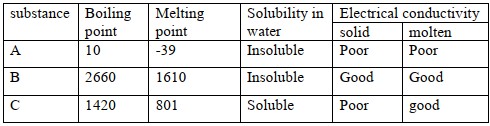
with reasons, select a substance
- with an electrovalent bond
- Likely to be a metal
Answer
- C - has high m.p and B.P and only conducts electricity in molten state.
- B - conducts electricity in both solid and molten state and has high m.p and B.P
Question 5
The use of materials of lead in roofing and in water pipes is being discouraged. State
- One reason why these materials have been used in the past.
Answer
- Lead piping had unique ability to resist pin hole leaks.
- lead does not rust/corrode forms a protective layer on its surface
- lead does not rust/corrode forms a protective layer on its surface
