Question 1
The pH values of some solutions labeled E to I are given in the table below. Use the information to answer the questions that follow.

- Identify the solution with the highest concentration of hydroxide ions. Explain
- Which solution can be used as a remedy for acid indigestion in the stomach? Explain
- Which solution would react explosively with Potassium metal?
Answer
- E, Strong base
- G, Magnesium Hydroxide
- F, Dilute acids reacts with Alkali metals Explosively
Question 2
Distinguish between ionization energy and electron affinity.
Answer
Ionization energy is the energy required to lose/donate an electron in an atom of an element in its gaseous state while electron affinity is the energy required to gain/acquire extra electron by an atom of an element in its gaseous state.
Electron affinity is the energy required to gain an electron in an atom of an element in gaseous state.
Electron affinity is the energy required to gain an electron in an atom of an element in gaseous state.
Question 3
The table below shows first ionization energies of metals represented by letters A, B, C and D. The metals are in the same group of the periodic table.
| Metal | A | B | C | D |
|---|---|---|---|---|
| 1st ionization energy (kJ/mole) | 402 | 496 | 520 | 419 |
Answer
C, has the smallest atomic radius, hence stronger nuclear force of attraction holding the outermost electron.
Question 4
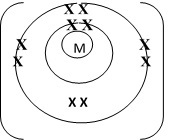
An element is represented as
- To which chemical family does it belong?
- Write the electron arrangement of the atom.
- Draw the structure of its ion.
Answer
- Alkali metals
- 2.8.1
Question 5
Define electrolysis.
Answer
Process by which an electrolyte gets decomposed when an electric current is passed through it.
