Question 1
State Charles' law.
Answer
The volume of a fixed mass of a gas is directly proportional to its absolute temperature at a constant pressure.
Question 2
A gas R at 27oC and 750mmHg was found to occupy 36cm3. calculate the temperature at which the same mass of R will occupy twice the volume at a pressure of 1000mmHg.
Answer
Question 3
Element A and B with atomic numbers 12 and 17 respectively react together.
- Write the electronic configurations of each
A -
B - - Write the formula of the compound formed between A and B
Answer
- Write the electronic configurations of each
A - 2:8:2
B - 2:8:7 - AB2
Question 4
The structure of water molecule can be represented as shown below.
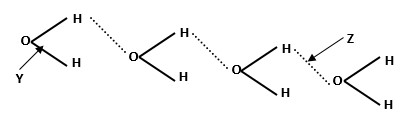
- Name the bond type represented by letters Y and Z.
Y
Z
- Methane and water are molecular substances with almost similar molecular masses however; the boiling point of water is 100oC while that of methane is –161oC. Explain
Answer
- Y - Covalent bond
Z - Hydrogen bond
- Bonding between water molecules is hydrogen bonding while bonding between methane molecules is weak vanderwaals forces. Hydrogen bonds are stronger than vanderwaals forces.
Question 5
The table below shows elements in the same group of the periodic table. Study it and answer the questions that follow.
| Element | Atomic size |
|---|---|
| B1 | 0.18 |
| B2 | 0.24 |
| B3 | 0.16 |
Which element has the highest ionization energy? Give a reason.
Answer
B3. Has the smallest atomic radius hence valency electrons are strongly attracted by positive nucleus hence requires more energy to remove.
