Question 1
The diagram below shows part of Solvay process.
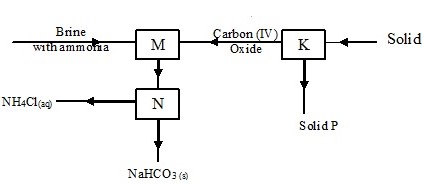
- Name solid P
- State the process taking place in chamber N
- State two uses of calcium chloride which is a by-product in this process.
Answer
- Calcium oxide
- Filtration
- 1. In the extraction of sodium metal
2. Pickling
Question 2
100cm3 of methane gas diffused through a porous partition in 40 seconds. How long would it take 90cm3 of ozone gas to diffuse through the same partition?
C = 12, H = 1, O = 16
Answer
Question 3
Ammonia is produced in large scale by Haber process.
- Write an equation for the formation of ammonia gas.
- State two optimum conditions for obtaining a high yield of ammonia in the process.
Answer
- N2 + 3H2(g) → 2NH3
- 1. Pressures of 200 atms
2. Temperature of 450°C to 500°C
Question 4
The scheme below shows some reactions starting with ethyne. Study it and answer the questions that follow.
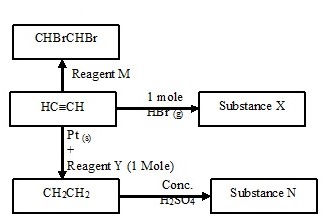
- Name substance X and N
- Name reagent M.
- Ethene undergoes polymerization to form a polymer. Name the product.
Answer
- X - Bromo ethene
N - Ethyl hydrogen sulphate - M - Bromine gas
- Name - Polyethene
Question 5
The curves below represent the volume of carbon (IV) oxide gas evolved once 2M(concentrated) hydrochloric acid was reacted with 100g of powdered calcium carbonate and also when 1M concentrated hydrochloric acid was reacted with the same quantity of carbonate.
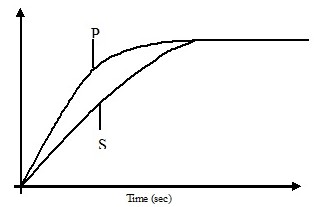
- Which of the two curves represents the reaction of 2M concentrated HCl with powdered calcium carbonate. Give a reason.
- Why do the two curves flatten at the same level of production of CO2
Answer
- P - It reacts with the carbonate faster and the reaction ends earlier.
- The same quantities of reactants have been used hence total volume of gas evolved is the same.
