Question 1
Give the systematic names of the following hydrocarbons
- CH3(CH2)4CH3
-
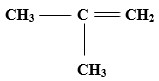
Answer
- Hexane
- 2-methyleprop-1-ene
Question 2
The molecular formula of a hydrocarbon is C6H14
The hydrocarbon can be converted into two other hydrocarbons as shown by the equation below.
C6H14 → X + C3H8
- What is the name of above process?
- Write the molecular formula of X
Answer
- Cracking
- C3H6
Question 3
Mr. Rudisha went to a doctor who sent him to a pharmacy to pick some drugs. The pharmacist wrote on the medicine packaging 2 x 3.
- Clearly state what 2 x 3 meant?
- State one reason why it is important to adhere to the doctor’s prescription
Answer
- Two tablets three times a day at interval of eight hours.
- To avoid overdose or underdose.
Question 4
The apparatus below were used by a student to study to effect of heat on hydrated copper (II) Sulphate.
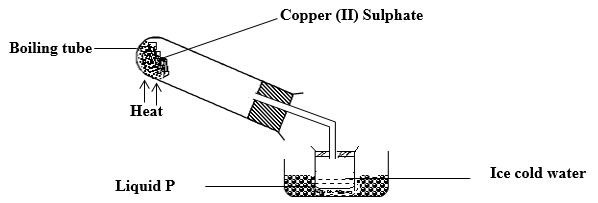
- What is the role of the ice-cold water?
- Name liquid P.
- What is the observation made in the boiling tube.
- Name type of change illustrated above
Answer
- To condense water vapour to liquid water.
- Water.
- Blue hydrated CuSO4 changes to white CuSO4 powder.
- Temporary chemical changes.
Question 5
The diagram below shows a paper chromatogram of substances A, B and C which are colored.
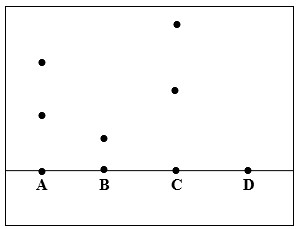
- Indicate baseline on the chromatogram.
- Which substance is pure? Explain
- Substance D is a mixture of B and C. Indicate it chromatogram on the diagram
Answer
- & c.
-h6BTaFLzgrHtXbDk.jpg)
- B; only one spot
