Question 1
Explain briefly how you would obtain crystals of sodium chloride from a mixture of sodium chloride, lead (II) chloride and iron fillings.
Answer
- Hold a magnet on top of the mixture to attract iron filling.
- Add water to the mixture, stir and filter to obtain PbCl2 as a residue and NaCl solution as the filtrate.
- Heat the filtrate to evaporate water leaving behind NaCl crystals
- Add water to the mixture, stir and filter to obtain PbCl2 as a residue and NaCl solution as the filtrate.
- Heat the filtrate to evaporate water leaving behind NaCl crystals
Question 2
What is meant by allotropy?
Answer
Allotropy is the existence of a substance in two or more forms without a change of state.
Question 3
The diagram below shows the structure of one of the allotropes of carbon
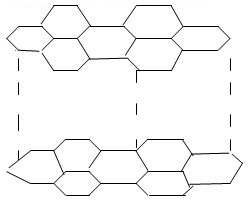
- Identify the allotrope.
- State one property of the above allotrope and explain how it is related to its structure.
Answer
- Graphite
- Conducts electricity, contains delocalised electrons. OR soft and slippery Hexagonal layer are held together by weak Van der Waals forces.
Question 4
- Using dots and cross diagram, show how a hydro-oxonium ion, H3O+ if formed
Hint: H2 + H+ → H3O+ (H=1, O=8) - What name is given to the bonding in (a) above.
Answer
-
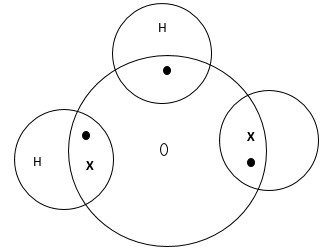
- Dative/coordinate bond
Question 5
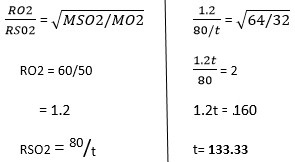
60cm3 of oxygen gas diffused through a porous hole in 50 seconds. How long will it take 80cm3 of sulphur(IV) oxide to diffuse through the same hole under the same conditions.
(S=32.0, O=16.0)
Answer