Question 1
Study the data in the table below and answer the questions. The letters do not represent the actual symbols of the elements.
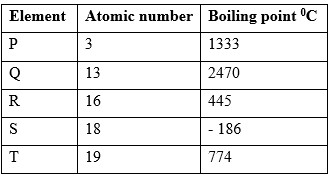
- Select the elements that belong to the same
(i) Group
(ii) Period - Write the;
(i) is a gas at room temperature? Explain
(Assume room temperature is 250C)
(ii) Does not take an oxide. - Which element
(i) Formula of the sulphate of element Q
(ii) Equation for the reaction between elements P and R. - What type of bonding would exist in the compound formed when elements R and Q react? Give a reason for your answer.
- Select the most electropositive element
- Explain why the boiling point of element Q is higher that that of element P.
Answer
-
(i) Same group - P and T
(ii) Same period - Elements Q, R and S -
(i) S - its boiling point is below room temperature.
(ii) Element S - it has a stable noble gas configuration and thus does not react. -
(i) Q2(SO4)3
(ii) P(s) + R2(g) → P2R - Ionic / electrovalent - there will be complete transfer of electrons from one element Q to another R .
- Element T
- Q has more valence electrons than P hence stronger metallic bond.
Question 2
In order to determine the molar heat of neutralization of potassium hydroxide, 200cm3 of 1M hydrochloric acid both at the same initial temperature were mixed and stirred continuously with a thermometer. The temperature of the resulting solution was recorded after every 30 seconds until the highest temperature of the solution was attained. Thereafter, the temperature of the solution was recorded for a further two minutes
-
(i) Why was it necessary to stir the mixture of the two solutions?
(ii) Define molar heat of neutralization
(iii) H+ + OH- → H2O(l)
H3O+ + OH- → P2R - If the initial temperature for both solution was 24.50C and the highest temperature attained by the mixture was 30.90C.
Calculate the:
I. heat change for the reaction (specific heat capacity of the solution = 4.2Jg-1K-1 and the density of the solution = 1.0g/cm3)
II. Molar heat at neutralization of potassium hydroxide - Explain how the value of the molar heat of neutralization obtained in the experiment would compare with the one that would be obtained if the experiment was repeated using 200cm3 of 1M ammonium hydroxide instead of 1M potassium hydroxide.
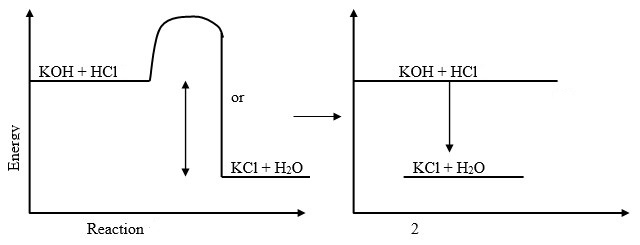
- On the grid provided below, draw an energy level diagram for the reaction between hydrochloric acid and potassium hydroxide.
Answer
-
(i) To distribute heat evenly / uniform mixture of reagents.
(ii) Molar heat of neutralization is the heat evolved when one mole of water is formed from the reaction between H+ and OH- ions.
(iii) H+ + OH- → H2O(l)
H3O+ + OH- → P2R -
I.
II.
- Molar heat will be lower because ammonium hydroxide is a weak base than potassium hydroxide/ ammonium hydroxide ionizes partially and some energy is used to ionize the remaining molecules.
-
Question 3
The reaction scheme below are some reactions starting with a brown solid X. Study the Scheme and answer the questions that follow.
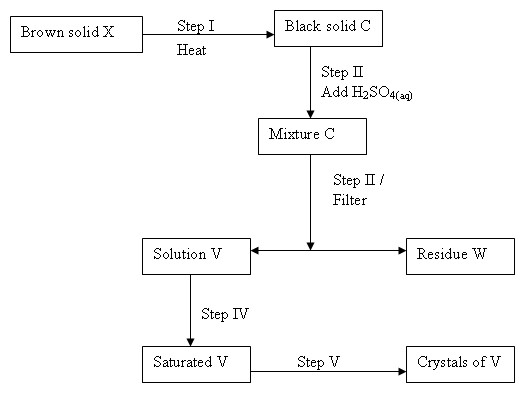
- Identify each of the following
(i) Solid X
(ii) Solid C
(iii) Mixture C
(iv) Solution V - (i) Write an equation for each of the reactions in steps I and II
(ii) Name the type of reaction in step II - Identify the reagent that was in excess in step II. Why should the reagent be in excess?
- State the condition for step II. Explain
- Describe how step V was carried out.
Answer
- (i) Solid X - Copper
(ii) Solid C - Copper II oxide
(iii) Mixture C - Copper II sulphate and excess copper II oxide.
(iv) Solution V - Copper II sulphate solution - (i) I. CuO(s) + O2(g) → CuO(s)
ii. CuO(s) + H2SO4(aq) → CuSO(aq) + H2O(l)
(ii) Neutralizations - Copper II oxide - it is easier to remove from the mixture than if the acid was inn excess.
- Warming / heating - to increase the rate of reaction by increasing the kinetic energy between the acid and copper oxide particles that are reaching.
- - Solution V is transformed into an evaporating dish the filtrate is evaporated over a water bath till saturation.
- The saturated solution is allowed to cool and crystallizes, the mixture is poured off, and the salt crystals are then dried between filter papers.
Question 4
In an experiment to study the rate of reaction between duralumin (an alloy of aluminium, magnesium and copper) and hydrochloric acid, 0.5g of the alloy were reacted with excess 4M Hydrochloric acid. The data in the table below was recorded. Study it and answer the questions that follow.
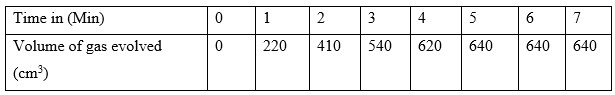
- Plot a graph of total volume of gas produced against time
- From the graph determine the volume of the gas produced at the end of minutes.
- From the graph determine the rate of reaction between 3rd and 4th minute.
- Give a reason why some solid remained at the end of the experiment.
Answer
- 490 ± 10cm3
- Part of duralumin does not react with the acid Since copper cannot react with dilute acid.
Question 5
Given that 2 – 5cm3 of the total volume of gas was from the reaction between the magnesium and hydrochloric acid. Calculate the percentage by mass of aluminium present in 0.5g of the alloy. (Al = 27.0, Molar gas volume = 24,000cm3 at 2981C).
Answer
2Al + 6HCl(aq) → 2AlCl3 + 3H2
Mole ration of Aluminium: Hydrogen = 2:3
∴ moles of aluminium = 0.0266146 x 2⁄3
= 0.0177431 moles
Mass of magnesium = 27 x 0.0177431
= 0.4790637g
Percentage by mass of aluminium
