Question 1
In experiment chlorine gas was passed into moist hydrogen sulphide in a boiling tube as shown in the diagram.

- State and explain the observations made in the boiling tube.
- Write an equation for the reaction that took place.
- What precaution should be taken when carrying out this experiment? Give a reason.
Answer
- A yellow solid is formed, chlorine oxidising agent. Oxidises H2S into sulphur
- Cl2(g) + H2S(g) → 2HCl(g) + S(s)
- Centred out in a fume chamber/ in open air. HCl produced is poisonus.
Question 2
State the type of bond in each of the following compounds.Study the scheme below and answer the questions that follow.
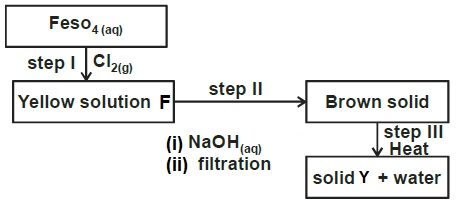
- Write the formula of the cation present in solution F.
- What property of chlorine is shown in step 1.
- Name the type of reaction in
- Step II
- Step III
- Give two uses of chlorine
Answer
- Fe3+
- oxidizing power.
-
- Step II - precipitation reaction
- Step III - Thermal decomposition
- 1. Used in manufacture of hydrochloric acid.
2. Manufacture of p.v.c. pipes
Question 3
Study the diagram below and answer the question that follow
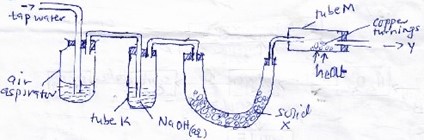
- What is the purpose of passing tap water through the air aspirator?
- State and explain the observation that would be made in tube M after sometime.
- Solid X changes to colourless solution at the end of the experiment. Name the process?
- A part from nitrogen gas , name another gas that may come out at point Y
- Write a chemical equation for the reaction that took place in tube K after the first two minutes.
Answer
- Push air through the apparatus.
- A black solid is formed. Copper combines with oxygen to form copper(II)oxide.
- Deliquescent
- Argon/excess oxygen
- 2NaOH(aq) + CO2 → Na2CO3(aq) + H2O(l)
Question 4
When a few drops of aqueous ammonia were added to colourless solution X, a white precipitate was formed and on addition of more aqueous ammonia, white precipitate dissolved to a colourless solution Q.
- Name the white precipitate formed.
- Write the formula of the complex ion present in the colourless solution Q.
- Write an ionic equation for the formation of the white precipitate.
Answer
- Zinc hydroxide
- Zn(NH3)42+
- Zn2+(aq) + 2OH-(aq) → Zn(OH)2(s)
Question 5
An oxide of nitrogen contains 28.2% nitrogen and the rest oxygen. Its molecular mass is 92, work out its molecular formula (0 = 16, N = 14).
Answer

