Question 1
The table below shows some elements in the periodic table. Use it to answer the question that follow. (The letter are not the actual symbols of the elements).
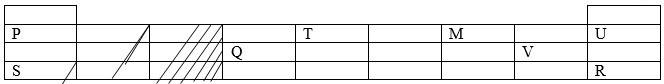
- Identify the elements in the same group.
- Give the name of the family to which elements P and S.
- Write the electron configuration of ions of elements;
i. V
ii. Q - Given that isotopes of element S are as follows 39S(93.1%), xS(0.01%) and 41S(6.89%), calculate x given that the relative atomic mass of element S is 39.1349.
- Elements Q and V react to form a compound.
i. Write an equation for the reaction
ii. What is the nature of the compound formed in (i) above. Explain
Answer
- P and S
U and R - Alkali metals
-
i. V- 2.8.8
ii. Q3+2.8 -
-
i. 2Q(s) + 3V2(g) → 2QV3
ii. Acidic. QV3 /AlCl3 hydrolyses in water to form H+ions, hence the acidic nature of the chloride
Question 2
The following set up was carried out by students in form two in the open air in the presence of sunlight to investigate a certain property of the halogen. Study it answer the questions.
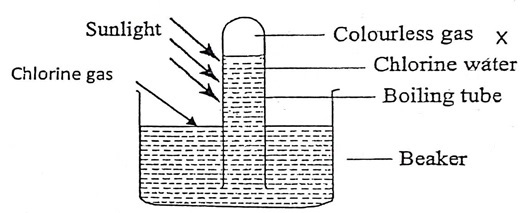
- Which property of the halogen was being investigated?
- Name the gas which was colourless
- Chlorine water is yellow in colour. However, in the presence of light it is decolourised. Explain
- Comment on the effect of chlorine water on red and blue litmus paper
Answer
- Solubility in water.
- Oxygen
- The yellow color is due to presence of chloric (I) acid, in the presence of light the chloric (I) acid decompose to form hydrochloric acid hence the solution turn colorless.
- Blue litmus paper turns red then both litmus papers are bleached to white. Chlorine water is acidic and has bleaching properties due to presence of chloric (I) acid / hypochlorous acid.
Question 3
The following diagram represents a section of the plant for the large scale manufacture of hydrochloric acid.
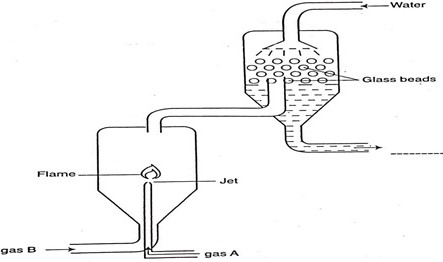
- Name gases A and B.
- State the role of glass beads in the plant
- Explain why gas A is introduced into the reaction chamber through a jet.
- Write the chemical equation for the reaction between gas A and B.
- Determine the volume of hydrochloric acid gas formed when 3600cm3 of gas B react with gas A at stp (MGV at stp = 22.4dm3)
Answer
- A - Hydrogen
B - Chlorine - To increase the surface area over which the gas dissolves in water.
- To prevent an explosion since the mixture of chlorine and hydrogen react explosively.
- H2(g) + Cl2(g) → 2HCl(g)
-
Question 4
CFC’S and DDT are chlorine compounds with long life span and so affects both plants and animal life. Write their full names.
CFC
DDT
Answer
CFC - Chlorofluorocarbons
DDT - Dichlorodiphenytrichloroethane
DDT - Dichlorodiphenytrichloroethane
Question 5
Butane and bromine react according to the equation below.
CH3CH2CH2CH3 + Br2 → CH3CH2CH2CH3Br2 + HBr
a) Name the type of reaction taking place in the equation above.
b) State the condition under which the above reaction takes place. Explain
Answer
a) Substitution
b) U.V light/ sunlight; presence of sunlight splits the halogen (bromine) molecules into free atoms which are very reactive hence they replace the hydrogen atoms in an alkane.
b) U.V light/ sunlight; presence of sunlight splits the halogen (bromine) molecules into free atoms which are very reactive hence they replace the hydrogen atoms in an alkane.
