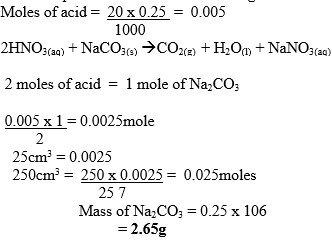Question 1
Below is a Bunsen burner flame.
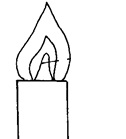
- Describe how this type of flame is produced.
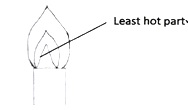
- Label on the diagram the least hot part of the flame.
- Name the gas produced by a burning candle that is a non-pollutant.
Answer
- By opening the air hole completely
-
- Water vapour (steam)
Question 2
The element x can be represented as
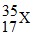
- How many neutrons are contained in X?
- Use the data in the table below to calculate the relative atomic mass of X from the masses and percentage abundance.
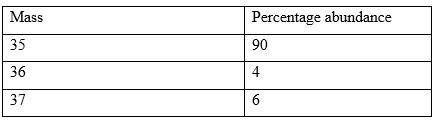
Answer
- 35 - 17 = 18
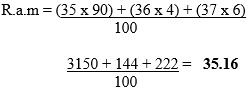
Question 3
- Write a chemical equation for the reaction that occur when Carbon (IV) oxide gas is bubbled in calcium hydroxide solution.
- Give a reason why Potassium hydroxide solution is not used to identify carbon (IV) oxide in the laboratory.
Answer
-
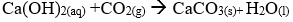
- It forms a colourless solution of potassium carbonate hence no visible change.
Question 4
Study the diagram below and answer the questions that follow.
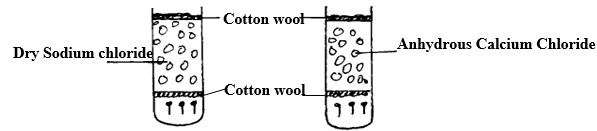
- State and explain the observations made after two weeks.
- Give one reason for Silver plating an Iron spoon.
Answer
- - Rusting occurred in tube (I). No rusting in (II).
- In tube (II) anhydrous calcium chloride absorbed moisture but not sodium chloride - - To improve its appearance /make it beautiful
- To prevent its corrosion or rusting
Question 5
In a neutralization reaction, Sodium carbonate solution was dissolved in water and the solution made to 250cm3. 25cm3 of this solution neutralized 20cm3 of 0.25Mnitric acid. Calculate the mass of carbonate that wasdissolved in water.
Answer