Question 1
A glass tube was inserted into a flame formed when the air hole of the Bunsen burner was fully open in the diagram below.

- When a burning splint was brought near point P, a small flame lit at this end of the glass tube. Explain
- Give two reason why the Bunsen burner flame above is preferred for heating than the flame obtained when the air hole is closed.
Answer
- Because the almost colorless region of a non-luminus flame contains the unburnt gases which will continue burning at the end of the glass tube when ignited.
- 1. Non-luminus flame prodeces much heat (it is the hottest)
2. The flame does not produce soot.
Question 2
- Using the Brousted – Lowly theory, define:
- An acid
- A base
- Given this reaction
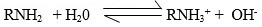
Identify the acid in the forward reaction
Answer
- An acid is a proton donor
- A base is a proton acceptor.
- H2O is the acid.
Question 3
State Graham’s Law of diffusion.
Answer
Graham’s Law of diffusion state the rate of diffusion of a gas is immersly proportional to the squareroot of its density provide the temperature and pressure are constant.
Question 4
A volume of 50cm3 of oxygen gas diffuse through a porous plug in 80 seconds. How long will it take 150cm3 of sulphur (iv) oxide gas to diffuse through the same plug. (S=32, O=16).
Answer
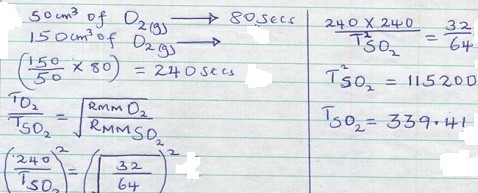
Question 5
The chromatogram of two inks and three dyes is drawn below;

- Name the colors of link A
- Suggest how separated components can be recovered.
- Suggest two reasons why separations occur in this method.
Answer
- Red and Blue
- By solvent extraction
- 1. Unequal solubilities
2. Different absorption abilities
