Question 1
a) Explain why non-luminous flame is preferred for heating.
b) State the role of colar in a Bunsen Burner.
Answer
a) - It produces a lot of heat due to complete combustion.
- It does produce suit that make apparatus dirty
b) It regulates the amount of air entering the chimney.
- It does produce suit that make apparatus dirty
b) It regulates the amount of air entering the chimney.
Question 2
In the solvay process calcium oxide reacts with water in the slaker to form calcium hydroxide.
a) Write the equation for the reaction.
b) State one role of calcium hydroxide in the laboratory.
c) Identify the by-product in the process which is not recycled.
Answer
a) CaO(s) + H2O(1) → Ca(OH)2
b) To test for presence of carbon (IV) oxide
c) Calcium chloride (CaCl2)
b) To test for presence of carbon (IV) oxide
c) Calcium chloride (CaCl2)
Question 3
3. The diagram below shows a chromatogram obtained when brands A and B of sodas were tested for a particular banned preservative
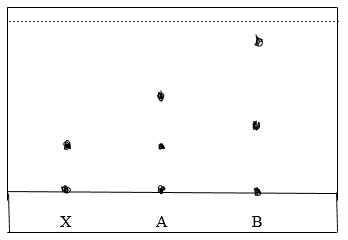
a) Identify the brand that contained banned preservative.
b) Show the solvent front on the diagram
c) Apart from the propanone, state other solvent that is used
Answer
a) A
b)-Qe1sG7q8eyd6EG1C.jpg)
c) – Ethanol - Methanol
b)
-Qe1sG7q8eyd6EG1C.jpg)
c) – Ethanol - Methanol
Question 4
a) State Boyle’s law
b) A given sample of oxygen gas occupies 16cm3 at 700 and 335 mmHg pressure. At what temperature will it occupy 10cm3 at 670mmHg pressure?
Answer
a) Boyle’s law - The volume of a given mass of a gas is inversely proportional to it’s pressure at constant temperature.
b)
b)
Question 5
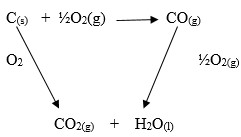
Calculate the heat of formation of carbon (II) oxide from the following data.
C(s) + O2(g) → CO2(g) ∆H1 = -394Kj/mol
C(s) + ½O2(g) → CO2(g) ∆H1 = -285.6
Answer