Question 1
The electron arrangement of ions X+, Y2+ and W3- are 2.8, 2.8 and 2.8.8 respectively.
a) Write the electron arrangement of their atoms.
b) Arrange the atoms in the order of increasing atomic radius starting with the smallest. Give a reason for the order.
Answer
- X 2.8.1
Y 2.8.2
W 2.8.5 - W Y X. Atomic radius decrease with increase in number of protons and nuclear charge.
Question 2
The diagram below shows a Bunsen burner when in use.
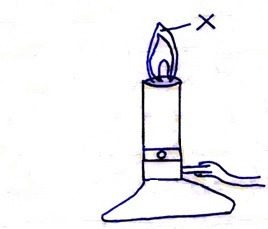
- State the condition under which the Bunsen burner produces the flame shown in the diagram above.
- Describe an experiment that can be carried out to confirm that the region labeled X is the hottest.
Answer
- When the air hole is open.
- Slip a white manila paper or wooden splint and quickly remove before it catches fire. The middle part burns uniformly.
Question 3
- Chlorides of Sodium and aluminium are given in the table below. Complete the table by writing the properties of the chlorides.
Property NaCl AlCl3 Bonding Structure - Sodium carbonate powder were added to aqueous solution of aluminium chloride. State and explain the observation made.
Answer
-
Property NaCl AlCl3 Bonding Ionic Covalent Structure Giant ionic Molecular - There is effervescence Aluminium chloride hydrolyses in water forming acidic solution.
Question 4
Explain why molten Magnesium Chloride conducts electric current while sugar solution do not.
Answer
Molten magnesium chloride has mobile ions while sugar solution has molecules and lack mobile ions.
Question 5
Complete the table below by writing the observations, anode and cathode half-equations for electrolysis of molten Lead (II) Chloride.
| Anode | Cathode | |
|---|---|---|
| Observation | ||
| Half-equations |
Answer
| Anode | Cathode | |
|---|---|---|
| Observation | Green-yellow gas | Grey solid |
| Half-equations | 2Cl-(l) → Cl2 + 2e | Pb2+(l) + 2e → Pb(s) |
