Question 1
a) State the structural difference between a thistle funnel and a dropping funnel.
b) List any two-apparatus used to measure accurate but specific volume of liquids in the lab.
Answer
a) A dropping funnel has a tap while a thistle does not have a tap.
b) - Pipette
- Volumetric flask
b) - Pipette
- Volumetric flask
Question 2
State the function of each of the following apparatus
a) Crucible
b) Desiccator
c) Wash bottle
d) Spatula
Answer
a) Crucible - for substances that require strong heating in the lab
b) Desiccator - to keep moisture away from substances
c) Wash bottle - to deliver liquids carefully into vessels with narrow necks
d) Spatula - to scoop solid substances from containers
b) Desiccator - to keep moisture away from substances
c) Wash bottle - to deliver liquids carefully into vessels with narrow necks
d) Spatula - to scoop solid substances from containers
Question 3
Draw the shapes of the following laboratory apparatus
a) Deflagrating spoon
b) Filter funnel
Answer
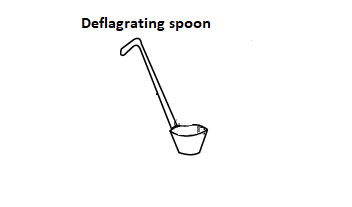
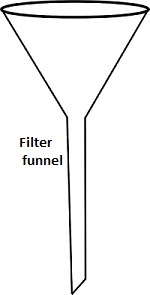
Question 4
Study the table below and answer the questions that follow.
| solution | pH |
|---|---|
| Q | 6.5 |
| R | 8 |
| S | 13 |
| T | 4 |
| V | 2 |
| W | 7 |
a) Choose a solution that is most likely to be;
I. Sodium chloride
II. Used as an anti-acid to relieve a heart burn
b) What color would methyl orange indicator have when added to the following solutions
I. Solution W
II. Solution T
c) Select a solution that would show the most vigorous effervescence when added a few grams of calcium carbonate.
d) Name the indicator that was used to obtain the PH values above
Answer
a) I. Sodium chloride - W
II. Used as an anti-acid to relieve a heart burn - R
b) I. Solution W - Orange
II. Solution T - Red
c) Solution T
d) Universal indicator
II. Used as an anti-acid to relieve a heart burn - R
b) I. Solution W - Orange
II. Solution T - Red
c) Solution T
d) Universal indicator
Question 5
a) With the aid of a well labeled diagram, show that the almost colorless region of a non-luminous flame consist of unburnt gases.
Answer
a) 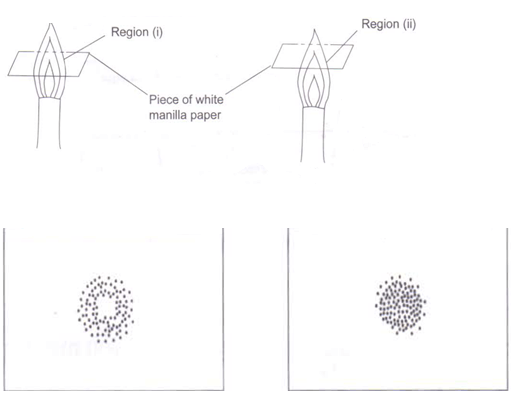
b) I. Close the air hole
II. Light the match stick and place it at the mouth of the chimney
III. Open the gas source
IV. Open the air hole

b) I. Close the air hole
II. Light the match stick and place it at the mouth of the chimney
III. Open the gas source
IV. Open the air hole
