Question 1
Study the figure below and answer the questions that follow.
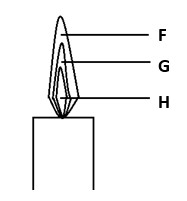
a) Name the parts labeled F and G
b) Give one reason why luminous flame is not used for heating purposes in the laboratory.
Answer
a) F – Thin outer region
G – Colourless region
b) Produces soot which makes apparatus dirty.
G – Colourless region
b) Produces soot which makes apparatus dirty.
Question 2
Describe how a sample of pure Sodium Chloride can be obtained from a mixture of Iodine, Sodium Chloride and sand.
Answer
Heat the mixture in an evaporating dish for iodine to sublime. Add enough water to the remaining mixture, stir, filter and heat the filtrate to dryness.
Question 3
a) Using dot (.) and Cross (x) show bonding in:
ion ( N=7,H-1.0)
b) State the type of bond that exists between the NH3 and H+ ion.
c) Give one reason why molecular substances have low melting points.
Answer
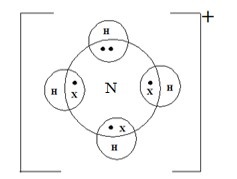
b) Dative | coordinate bond
c) The molecules are held by weak Van der waals forces.
Question 4
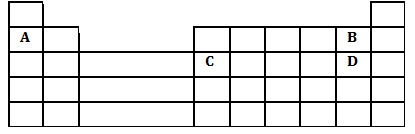
A part of the periodic table is given below. Study it and answer the questions that follow.
Answer
i. What type of bond is formed when A reacts with D? Explain.
ii. Explain the difference in the atomic radii of element C and D.
iii. Explain the difference in the reactivity of B and D.
ii. Explain the difference in the atomic radii of element C and D.
iii. Explain the difference in the reactivity of B and D.
Question 5
In the industrial preparation of Oxygen, state:
a) How dust particles are removed from air.
b) Why carbon (IV) oxide is removed before the mixture is cooled to -25oC.
Answer
a) By passing the air through filters.
b) Carbon (IV) oxide would otherwise solidify and block the pipes.
b) Carbon (IV) oxide would otherwise solidify and block the pipes.
