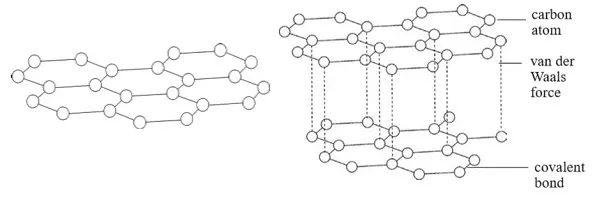
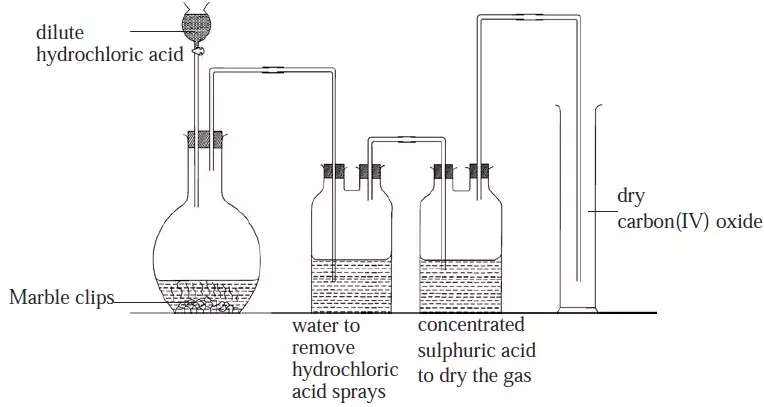
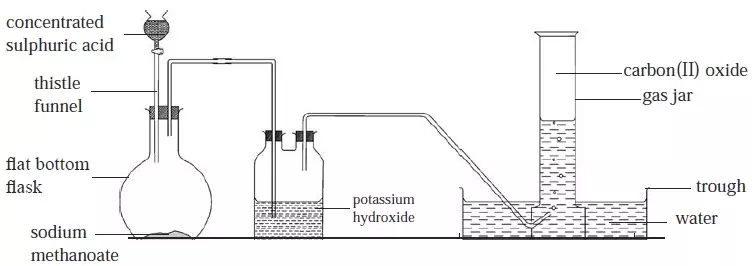
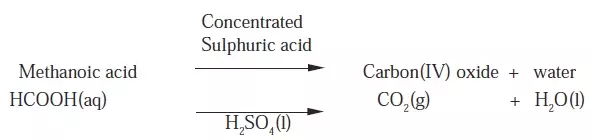
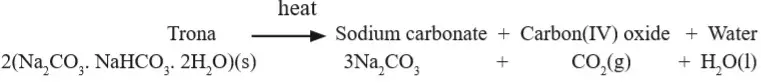- It will be observed that some carbonates decompose when heated and others do not.
- Carbonates of:

- (NH
4)
2CO
3 produces carbon(IV) oxide, water and ammonia gas on heating.
- Therefore, the carbonates of metals high in the reactivity series do not decompose.
- Ease of decomposition of metal carbonates increases as we go down the reactivity series.
- The following are equations showing decomposition of carbonates.
Copper carbonate → copper(II) oxide + carbon(IV) oxide
CuCO
3(s)
(green) → CuO(s)
(black) + CO
2(g)
Magnesium carbonate → magnesium(II) oxide + carbon(IV) oxide
MgCO
3(s)
(white) → MgO(s)
(white) + CO
2(g)
Zinc carbonate → zinc oxide + carbon(IV) oxide
ZnCO
3(s)
(white) → ZnO(s)
(white) + CO
2(g)
Lead carbonate → lead(II) oxide + carbon(IV) oxide
PbCO
3(s)
(white) → PbO(s)
(yellow)) + CO
2(g)
Calcium carbonate → calcium oxide + carbon(IV) oxide
CaCO
3(s)
(white) → CaO(s)
(white) + CO
2(g)
- Copper(II) oxide is black, while oxides of magnesium and calcium are white.
- Zinc oxide is yellow when hot and white on cooling.
- Lead(II) oxide is red-brown when hot and yellow on cooling.
- Sodium carbonate and potassium do not decompose on heating.
- Sodium hydrogencarbonate and calcium hydrogencarbonate decompose on heating to form a carbonate, water and carbon(IV) oxide.
Sodium hydrogencarbonate → sodium carbonate + carbon(IV) oxide + water
2NaHCO
3(s) → Na
2CO
3(s) + CO
2(g) + H
2O(l)
Calcium hydrogencarbonate → calcium carbonate + carbon(IV) oxide + water
Ca(HCO
3)
2(s) → CaCO
3(s) + CO
2(g) + H
2O(l)
- When calcium hydrogencarbonate is heated strongly calcium carbonate formed decomposes to form calcium oxide and carbon(IV) oxide.
- Ammonium carbonate decomposes on heating to form ammonia gas, water and carbon(IV) oxide.
Ammonium carbonate → ammonia + carbon(IV) oxide + water
(NH
4)
2CO
3(s) → 2NH
3 (g) + CO
2(g) + H
2O(l)
- Ammonia and carbon(IV) oxide gases are liberated at the same time.
- If moist red and blue litmus papers are put together at the mouth of the test-tube when ammonium carbonate is heated, ammonia gas is detected first
red litmus paper turns blue.
Note: Sometimes sodium carbonate may be contaminated with sodium hydrogencarbonate and therefore carbon(IV) oxide can be obtained on heating it.
Action of Dilute Acids on Carbonates and Hydrogencarbonates
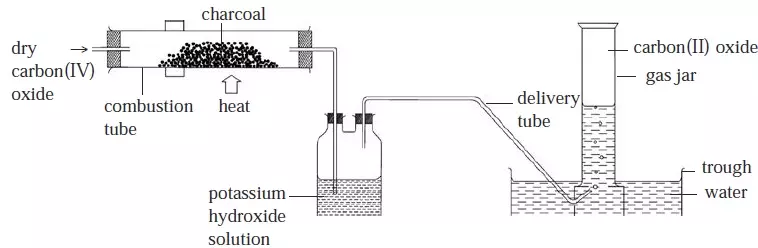
- All carbonates liberate carbon(IV) oxide on addition of a dilute acid.
- They also form a salt and water. For example:
Calcium carbonate + nitric acid → calcium Nitrate + water + carbon(IV) oxide
CaCO
3(s) + 2HNO
3(aq) → Ca(NO
3)(aq) + H
2O(l) + CO
2(g)
Calcium carbonate + hydrochloric acid → calcium chloride + water + carbon(IV) oxide
CaCO
3(s) + 2HCl(aq) → CaCl
2(aq) + H
2O(l) + CO
2(g)
All hydrogencarbonates also liberate carbon(IV) oxide on addition of an acid. For example:
Sodium hydrogen carbonate + nitric acid → sodium nitrate + carbon(IV) oxide + water
NaHCO
3(s) + HNO
3(aq) → NaNO
3(aq) + CO
2(g) + H
2O(l)
Sodium hydrogencarbonate + hydrochloric acid → sodium chloride + carbon(IV) oxide + water
NaHCO
3(s) + HCl(aq) → NaCl(aq) + CO
2(g) + H
2O(l)
Production and Manufacture of Sodium Carbonate
I. Production of sodium carbonate (soda ash) at Magadi Soda Company
- The most efficient means of trona extraction uses
dredging techniques.
- Both a
bucket dredge and a
cutter-suction dredge are used for this purpose, concentrating on the top 4 metres or so of the solid crust where the purest material is found.
- As the solid material is removed from the surface,
liquor (unwanted solution) from the surrounding trona drains into the resulting cavity, forming a pool or
paddock in which dredgers can float.
- The crystals of trona produced by the dredgers are mixed with liquor and pumped as slurry back to the ash plant.
- Here the liquor is discarded and the crystal washed and centrifuged.
- The dump crystals are then fed into
calciners (kilns).
- Here the residual moisture, water crystallisation and carbon(IV) oxide gas are driven off to leave normal sodium carbonate (soda ash).
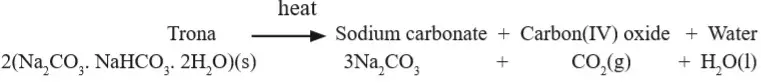
- The soda ash from the calciners passes through rotary drum coolers before entering the grinding and screening plant.
- Oversize material is removed and grounded again.
- The final product is conveyed to silos where it is packed or loaded directly into specially bulk raid hopper wagons.
- The liquor from this process is used to extract sodium chloride.
- Solar evaporation process is used. Most of the Magadi sodium chloride is sold within Kenya for livestock consumption or for industrial purposes.
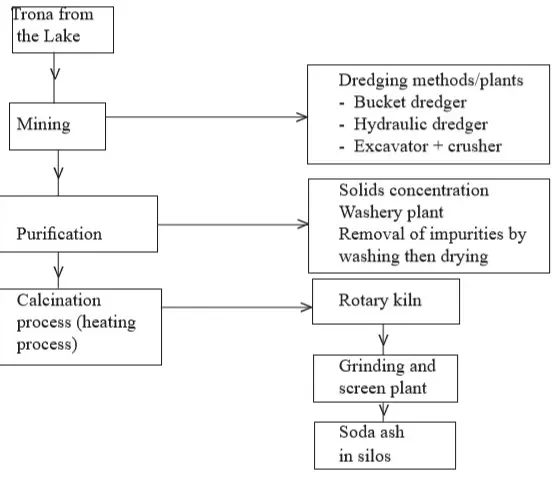
Figure 9: A flow chart of soda ash manufacturing process
II. Manufacture of sodium carbonate (soda ash) by Solvay process
- The main raw materials are sodium chloride and calcium carbonate.
- Concentrated brine (sodium chloride solution) is saturated with ammonia in a tower.
- The ammoniacal brine formed is run down the Solvay tower.
- Carbon(IV) oxide is forced into the tower from the bottom.
- The towers are filled with baffles. These baffles make the liquid flow slowly and increase the surface area for reaction.
- Sodium hydrogen carbonate is formed in Solvay tower. The reaction occurring is as follows.
Sodium chloride + ammonia + carbon(IV) oxide + water → sodium hydrogencarbonate + ammonium chloride
NaCl(g) + NH
3(aq) + CO
2(g) + H
2O(I) → NaHCO
3(s) + NH
4Cl(aq)
- Then sodium hydrogencarbonate is filtered off, and washed to remove ammonium chloride.
- It is then heated in a furnace roaster to give sodium carbonate and carbon(IV) oxide.
Sodium hydrogencarbonate → sodium carbonate + carbon(IV) oxide + water
2NaHCO
3(s) → Na
2CO
3(s) + CO
2(g) + H
2O(l)
- Carbon(IV) oxide formed is recycled to the Solvay tower.
- The other source of carbon(IV) oxide is from heating calcium carbonate(limestone) in the kiln where it dissociates into calcium oxide and carbon(IV) oxide.
Calcium carbonate → calcium oxide + carbon(IV) oxide
CaCO
3(s) → CaO(s) + CO
2(g)
- The calcium oxide formed from above reaction is
slaked by addition of water.
Calcium oxide + water → calcium hydroxide
CaO(s) + H
2O(l) → Ca(OH)
2z(s)
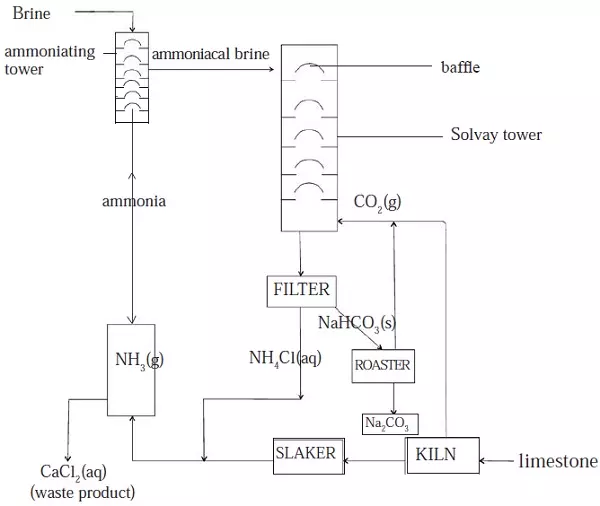
Figure 10: Solvay process
- The calcium hydroxide formed is heated with the filtrate, ammonium chloride from the solvay tower, to produce ammonia.
Ammonium chloride + calcium hydroxide → calcium chloride + ammonia + water
2NH
4Cl(aq) + Ca(OH)
2(s) → CaCl
2(aq) + 2NH
3(g) + 2H
2O(l)
- The ammonia from this reaction is returned to the ammoniating tower.
- A careful examination of the flow diagram (Figure 10) shows that the only waste product in this process is calcium chloride.
- The carbon(IV) oxide and ammonia are recycled.
- Therefore, the solvay process is very efficient.
- The raw materials are cheap and readily available and only one waste product is formed.
Uses of sodium carbonate
1. Manufacture of glass.
2. In domestic water-softening.
3. In the manufacture of chemicals e.g. sodium hydroxide.
4. In the manufacture of laundry detergents.
5. In the paper-making process.
6. Textiles.