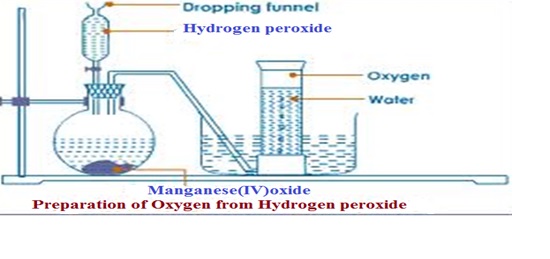
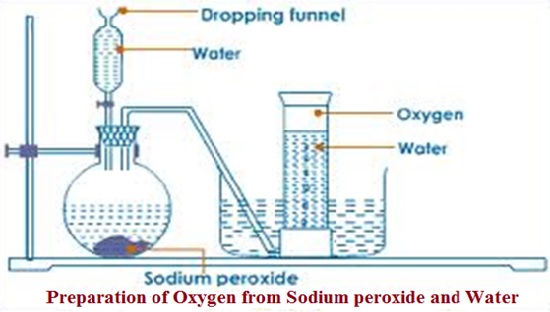
There are various gases in the atmosphere a listed below with their percentage composition;
- Nitrogen - 78%
- Oxygen - 21%
- Carbon (IV) Oxide - 0.03%
- Noble gases - 1%
- Water Vapour - varies by region
There are different experiments that could be used to demonstrate presence and composition of the above gases in air
Some of them include:
Finding the composition of air supporting combustion using a candle stick
ProcedureMeasure the length of an empty gas jar M1. Place a candle stick on a Petri dish. Float it on water in basin/trough. Cover it with the gas jar. Mark the level of the water in the gas jar M2. Remove the gas jar. Light the candle sick. Carefully cover it with the gas jar. Observe for two minutes. Mark the new level of the water M3. Set up of apparatus
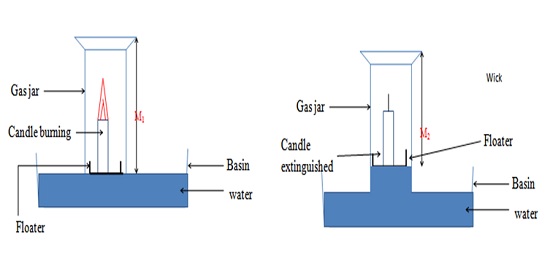
The observations are made because;
Candle burns in air. In a closed system (vessel), the candle continues to burn using the part of air that support burning/combustion. This is called the active part of air. The candle goes off/extinguished when all the active part of air is used up. The level of the water rises to occupy the space /volume occupied by the used active part of air
NOTE: The experiment is better when very dilute sodium/potassium hydroxide is used instead of water. Dilute Potassium/ sodium hydroxide absorb Carbon (IV) oxide gas that comes out from burning/combustion of candle stick.
From the setup above, the percentage composition of active part of air (oxygen) is calculated as follows;
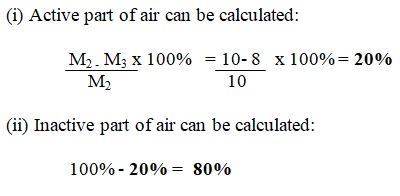
There is a host of experiments that can be done to find the composition of the active part of air (oxygen) as follows;
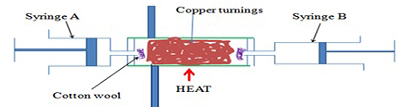
a) Using copper turnings as illustrated below
This is because copper reacts with oxygen to form Copper (II) Oxide
Copper + Oxygen  Copper(II)Oxide
Copper(II)Oxide
- The cotton wool in the experiment copper turnings from being blown into the syringe/out of the glass tube.
- Air is passed through the glass tube repeatedly to ensure all the active part of air is used up.
- Passing air through the glass tube slowly allows enough time of contact between the active part of and the heated copper turnings.
- Colour changes from brown to black due to the reaction of brown copper metal with oxygen to form copper (II) oxide.
- The reaction reduces the amount/volume of oxygen in syringe “B” leaving the inactive part of air, because copper only react with oxygen when heated.
- The percentage of active part of air is theoretically higher because not all the active part of air reacted with copper.
- If the copper turnings are replaced with magnesium shavings the % of active part of air obtained is extraordinary very high because magnesium is more reactive than copper, and the reaction is highly exothermic. Hence it generates enough heat for magnesium to react with both oxygen and nitrogen in the air.
Magnesium + Oxygen
 Magnesium (II) Oxide
Magnesium (II) OxideMagnesium + Nitrogen
 Magnesium (II) Nitride
Magnesium (II) Nitride
b. Using alkaline pyrogallol
In this test, the colour of pyrogallol/1, 2, 3-trihydroxybenzene change to brown.This is because oxygen gas is absorbed by alkaline pyrogallol/1,2,3-trihydroxybenzene.
c. Testing the presence of carbon (IV) oxide in air using lime water
Set up the experiment as shown below and pass tap water slowly into the empty flask.
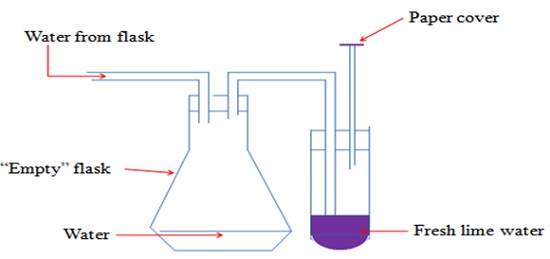
- The paper cover ensures that no air enters into the lime water.
- When water enters the flask, it forces the air from the flask into the lime water.
- A white precipitate is formed. The white precipitate dissolves on prolonged bubbling of air.
- Lime water is Calcium hydroxide / Ca(OH)2
- The white precipitate formed is Calcium carbonate/ CaCO3
Calcium hydroxide + carbon (IV) oxide
 Calcium carbonate + water
Calcium carbonate + waterCa (OH)2(aq) + CO2 (g)
 CaCO3(s) + H2O (l)
CaCO3(s) + H2O (l)- When the white precipitate dissolves, the solution formed is Calcium hydrogen carbonate/ CaHCO3
Calcium carbonate + water + carbon (IV) oxide
 Calcium hydrogen carbonate
Calcium hydrogen carbonateCaCO3(s) + H2O (l) + CO2 (g)
 CaHCO3 (aq)
CaHCO3 (aq)- Carbon (IV) oxide forms a white precipitate with lime water that dissolves in excess of the gas.