A base may be defined as a substance that turns litmus blue.
An indicator is a substance that shows whether another substance is a base/alkaline,acid or neutral
Acids
There are some common and naturally occurring acids as shown in the table below| Name of Acid | Occurrence |
|---|---|
| 1. Citric acid | Found in ripe citrus fruits like passion, oranges, and lemon |
| 2. Tartaric acid | Found in grapes/baking powder/health salts |
| 3. Lactic acid | Found in sour milk |
| 4. Ethanoic acid | Found in vinegar |
| 5. Methanoic acid | Present in ants and bees stings |
| 6. Carbonic acid | Used in preservation of fizzy drinks like coke, Lemonade and Fanta |
| 7. Butanoic acid | Present in cheese |
| 8. Tannic acid | Present in tea |
However, acids commonly used and found in a school laboratory are not naturally occurring
They are mineral acids as illustrated below
| Name of Mineral Acid | Common Use |
|---|---|
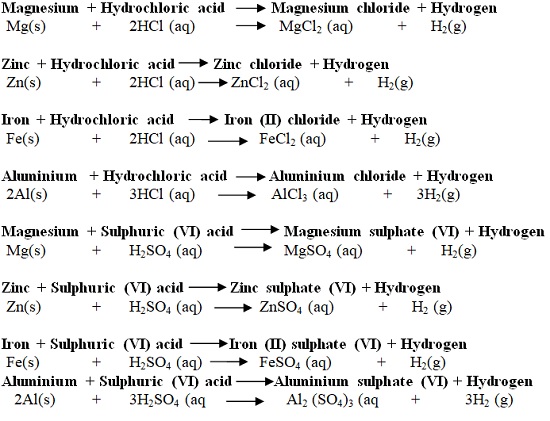
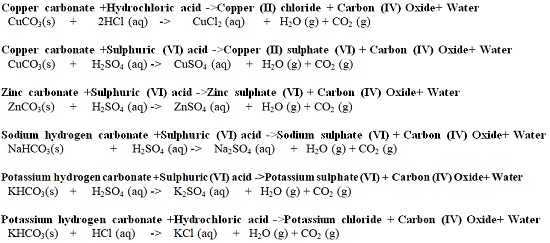
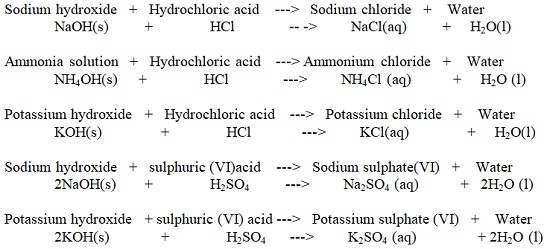
| Hydrochloric acid (HCl) | Used to clean/pickling surface of metals. It is found in the stomach of mammals/human beings and helps in digestion. |
| Sulphuric (VI) acid (H2SO4) | Used as acid in car battery, making battery and making fertilizers |
| Nitric (V) acid (HNO3) | Used in making fertilizers and explosives |
- They are corrosive (causes painful wounds on contact with the skin) and attack/reacts with garments/clothes/metals.
- In a school laboratory, they are mainly used when added a lot of water. This is called diluting. Diluting ensures the concentration of the acid is safely low.
Bases
Bases are opposite of acids.Most bases do not dissolve in water.
Bases which dissolve in water are called alkalis.
Some common alkalis and their uses include;
| Name of Alkali | Common Uses |
|---|---|
| Sodium Hydroxide (NaOH) | Making soaps and detergents |
| Potassium Hydroxide (KOH) | Making soaps and detergents |
| Ammonia Solution (NH4OH) | Making fertilizers and softening hard water |
Some common uses of bases that are not soluble in water (non-alkalis)
| Name of Non-Alkali | Common Uses |
|---|---|
| Magnesium Oxide/Hydroxide | Anti acid to treat indigestion |
| Calcium Oxide | Making cement and neutralizing soil acidity |
Indicators
- An acid-base indicator is a substance used to identify whether another substance is alkaline or acidic.- An acid-base indicator works by changing to different colors in neutral, acidic and alkaline solutions/dissolved in water.
- The following table shows the changes in color shown by indicators in different types of solution:
| Indicator | Acids | Base / Alkali | Neutral |
|---|---|---|---|
| Litmus Paper/Solution | Red | Blue | Colourless |
| Methyl Orange | Red | Yellow | Red |
| Screened Methyl Orange | Purple | Orange | Orange |
| Phenolphthalein | Colourless | Purple | Colourless |
| Bromothymol Blue | Orange | Blue | Orange |
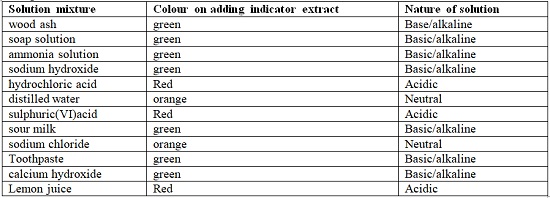
- Common indicators are used in school laboratories. They are cheap, readily available and easy to store. Common indicators include: Litmus, phenolphthalein, methyl orange, screened methyl orange, bromothymol blue.
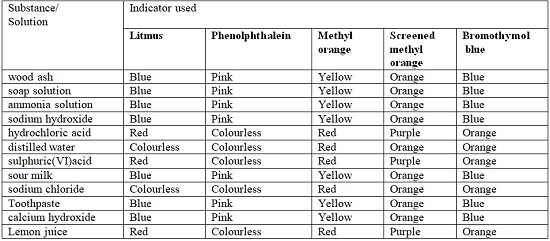
- The following table shows different results when indicators are used to test different solutions
The Universal Indicator
Universal indicator is a mixture of other indicator dyes. The indicator uses the pH scale that shows the strength of bases and acids in a range of 1-14 as follows:- pH values 1, 2, 3 shows a substance is a strongly acid
- pH values 4, 5, 6 shows a substance is a weakly acid
- pH value 7 shows a substance is a neutral
- pH values 8, 9, 10, 11 shows a substance is a weak base/alkali.
- pH values 12, 13, 14 shows a substance is a strong base/alkali
When determining the pH of a unknown solution using pH paper, the pH paper is dipped into the unknown solution. It changes/turn to a certain colour. The new colour is marched/compared to its corresponding one on the pH chart to get the pH value.
When determining the pH of a unknown solution using universal indicator solution, about 3 drops of the universal indicator solution is added into about 5cm3 of the unknown solution in a test tube. It changes/turn to a certain colour. The new colour is marched/compared to its corresponding one on the pH chart to get the pH value.